In Development this week (Vol. 141, Issue 9)
Posted by Seema Grewal, on 22 April 2014
Here are the highlights from the new issue of Development:
Hemogenic endothelium flexes some muscle
 Mesoangioblasts (MABs) are progenitor cells of embryonic derivation with mesodermal potential. They have been successfully used to restore skeletal muscle loss in dystrophic mice, but despite the clinical potential of these cells, their origin and role during development has not been defined. Now, on p. 1821, Silvia Brunelli and colleagues identify embryonic MABs that originate from the hemogenic endothelium during the early stages of mouse embryogenesis. The authors use a lineage tracing approach based on VE-cadherin expression to show that the MABs originate from endothelial cells (ECs) in the yolk sac and the placental tissues from approximately embryonic day (E) 8.5 until E10.5, and that these cells contribute to multiple mesodermal lineages during development, including skeletal muscle. The authors further show that this VE-Cadherin-positive extra-embryonic endothelium also generates the first wave of hematopoietic cells that colonise the embryonic mesenchyme. This study demonstrates for the first time that the embryonic hemogenic endothelium can generate extra-vascular mesodermal tissue in vivo.
Mesoangioblasts (MABs) are progenitor cells of embryonic derivation with mesodermal potential. They have been successfully used to restore skeletal muscle loss in dystrophic mice, but despite the clinical potential of these cells, their origin and role during development has not been defined. Now, on p. 1821, Silvia Brunelli and colleagues identify embryonic MABs that originate from the hemogenic endothelium during the early stages of mouse embryogenesis. The authors use a lineage tracing approach based on VE-cadherin expression to show that the MABs originate from endothelial cells (ECs) in the yolk sac and the placental tissues from approximately embryonic day (E) 8.5 until E10.5, and that these cells contribute to multiple mesodermal lineages during development, including skeletal muscle. The authors further show that this VE-Cadherin-positive extra-embryonic endothelium also generates the first wave of hematopoietic cells that colonise the embryonic mesenchyme. This study demonstrates for the first time that the embryonic hemogenic endothelium can generate extra-vascular mesodermal tissue in vivo.
Motor efferent axons lead the way
 The assembly of the peripheral nervous system occurs in a precise order: motor efferent axons (MEs) emerge first, followed by somatosensory afferent axons (SAs), and then by sympathetic efferent axons (SEs). While this order is clearly defined, it is not clear whether the pioneering axons provide instructive cues for the trailing axons to follow, and thus whether the network represents a true hierarchy. In this issue (p. 1875), Till Marquardt and colleagues take an evolutionary approach to address this issue, and find that peripheral nerve assembly is governed by a stringent hierarchy of axon-dependent interactions. Using elegant in vivo genetic analyses to manipulate sensory and motor axons networks in three different vertebrate organisms – fish, chick and mouse – the authors show that MEs act as pioneer axons, laying down tracks that are followed by SAs, which in turn act as pioneers for SEs. The authors argue that this hierarchy mirrors the phylogenetic emergence of peripheral nerve types during vertebrate evolution.
The assembly of the peripheral nervous system occurs in a precise order: motor efferent axons (MEs) emerge first, followed by somatosensory afferent axons (SAs), and then by sympathetic efferent axons (SEs). While this order is clearly defined, it is not clear whether the pioneering axons provide instructive cues for the trailing axons to follow, and thus whether the network represents a true hierarchy. In this issue (p. 1875), Till Marquardt and colleagues take an evolutionary approach to address this issue, and find that peripheral nerve assembly is governed by a stringent hierarchy of axon-dependent interactions. Using elegant in vivo genetic analyses to manipulate sensory and motor axons networks in three different vertebrate organisms – fish, chick and mouse – the authors show that MEs act as pioneer axons, laying down tracks that are followed by SAs, which in turn act as pioneers for SEs. The authors argue that this hierarchy mirrors the phylogenetic emergence of peripheral nerve types during vertebrate evolution.
On growth and gradients
 How does a developing tissue know how much to grow and when to stop? On p. 1884, Marcos Gonzalez-Gaitan and colleagues address this question using theDrosophila eye as a model. This study follows their earlier work proposing a temporal model for growth control in the wing, whereby cells divide when the levels of Decapentaplegic (Dpp) signalling increase by a defined percentage. In the eye, spatial growth patterns are very different from those in the wing, and growth is partially dependent on a Dpp gradient, the source of which – the morphogenetic furrow – moves as development progresses. The authors find that, as in the wing, the signal gradient scales with tissue size – which grows and then shrinks with the progression of the furrow. They then show that their temporal model is quantitatively consistent with observed patterns of proliferation in wild-type and in various mutant conditions. Intriguingly, they also show that the Dpp-independent component of growth control can be explained by a temporal model – implying a similar cellular response to a different signalling gradient. Thus, a model of tissue growth that involves cells dividing in response to defined increases in signalling levels may be applicable across multiple tissues and multiple signalling inputs.
How does a developing tissue know how much to grow and when to stop? On p. 1884, Marcos Gonzalez-Gaitan and colleagues address this question using theDrosophila eye as a model. This study follows their earlier work proposing a temporal model for growth control in the wing, whereby cells divide when the levels of Decapentaplegic (Dpp) signalling increase by a defined percentage. In the eye, spatial growth patterns are very different from those in the wing, and growth is partially dependent on a Dpp gradient, the source of which – the morphogenetic furrow – moves as development progresses. The authors find that, as in the wing, the signal gradient scales with tissue size – which grows and then shrinks with the progression of the furrow. They then show that their temporal model is quantitatively consistent with observed patterns of proliferation in wild-type and in various mutant conditions. Intriguingly, they also show that the Dpp-independent component of growth control can be explained by a temporal model – implying a similar cellular response to a different signalling gradient. Thus, a model of tissue growth that involves cells dividing in response to defined increases in signalling levels may be applicable across multiple tissues and multiple signalling inputs.
Developing concepts of wound healing
 Wound repair is a fundamental process that is required for tissue homeostasis and regeneration following damage. Most studies of wound healing have focussed on changes in the leading edge of wounded cells, but here William Razzell, Will Wood and Paul Martin show that morphogenetic cell shape changes that occur multiple cell rows back from the wound are important for efficient wound re-epithelialisation (p. 1814). Using laser-induced wounding of the Drosophila embryo epidermis as a model, the researchers first show that multiple rows of cells around the wound stretch towards the closing tissue. They further reveal dramatic shrinking of the cell-cell junctions that are perpendicular to the pulling force of the wound. This shrinking, which is driven by pulses of myosin that are directed to the cell junctions, leads to cell intercalations. Importantly, these morphogenetic changes, which resemble those observed during the developmental event of germband extension, are essential for wound closure; blocking myosin activity in cells behind the leading edge results in delayed wound contraction. This work highlights an important role for cells surrounding the wound in its closure, and suggests that the cellular morphogenetic mechanisms used during development are recapitulated during wound healing.
Wound repair is a fundamental process that is required for tissue homeostasis and regeneration following damage. Most studies of wound healing have focussed on changes in the leading edge of wounded cells, but here William Razzell, Will Wood and Paul Martin show that morphogenetic cell shape changes that occur multiple cell rows back from the wound are important for efficient wound re-epithelialisation (p. 1814). Using laser-induced wounding of the Drosophila embryo epidermis as a model, the researchers first show that multiple rows of cells around the wound stretch towards the closing tissue. They further reveal dramatic shrinking of the cell-cell junctions that are perpendicular to the pulling force of the wound. This shrinking, which is driven by pulses of myosin that are directed to the cell junctions, leads to cell intercalations. Importantly, these morphogenetic changes, which resemble those observed during the developmental event of germband extension, are essential for wound closure; blocking myosin activity in cells behind the leading edge results in delayed wound contraction. This work highlights an important role for cells surrounding the wound in its closure, and suggests that the cellular morphogenetic mechanisms used during development are recapitulated during wound healing.
miR-8 enables correct synaptogenesis
 Synaptogenesis is a complex process that involves the coordinated assembly of pre- and postsynaptic compartments. Various extracellular pathways and cues have been shown to regulate synapse formation but here, on p. 1864, David Van Vactor and colleagues show that the microRNA miR-8 controls synapse structure by repressing the actin regulator Enabled (Ena) and hence modulating synapse morphogenesis at the Drosophila neuromuscular junction (NMJ). The authors previously identified miR-8 as a potent regulator of NMJ architecture and presynaptic morphogenesis, and now find that Ena is direct target of miR-8 that is crucial for mediating its activity in synapse formation. Ena is enriched in the postsynaptic peribouton area surrounding the presynaptic compartment, and this localisation appears to depend on conserved actin-binding domains in the C-terminus of Ena. Further studies suggest that miR-8 controls NMJ architecture by inhibiting Ena expression and, hence, limiting the levels of postsynaptic Ena-dependent actin assembly, which in turn can regulate the expansion of presynaptic arbours. Together, these studies uncover a novel morphogenetic mechanism that coordinates the remodelling of pre- and post-synaptic compartments.
Synaptogenesis is a complex process that involves the coordinated assembly of pre- and postsynaptic compartments. Various extracellular pathways and cues have been shown to regulate synapse formation but here, on p. 1864, David Van Vactor and colleagues show that the microRNA miR-8 controls synapse structure by repressing the actin regulator Enabled (Ena) and hence modulating synapse morphogenesis at the Drosophila neuromuscular junction (NMJ). The authors previously identified miR-8 as a potent regulator of NMJ architecture and presynaptic morphogenesis, and now find that Ena is direct target of miR-8 that is crucial for mediating its activity in synapse formation. Ena is enriched in the postsynaptic peribouton area surrounding the presynaptic compartment, and this localisation appears to depend on conserved actin-binding domains in the C-terminus of Ena. Further studies suggest that miR-8 controls NMJ architecture by inhibiting Ena expression and, hence, limiting the levels of postsynaptic Ena-dependent actin assembly, which in turn can regulate the expansion of presynaptic arbours. Together, these studies uncover a novel morphogenetic mechanism that coordinates the remodelling of pre- and post-synaptic compartments.
Plus…
Actomyosin networks and tissue morphogenesis
 Tissue morphogenesis is driven by coordinated cellular deformations and recent studies have shown that these changes in cell shape are powered by intracellular contractile networks comprising actin filaments, actin cross-linkers and myosin motors. In their Development at a Glance poster article, Munjal and Lecuit provide an overview of the mechanics, principles and regulation of actomyosin-driven cellular tension driving tissue morphogenesis. See the article on p. 1789
Tissue morphogenesis is driven by coordinated cellular deformations and recent studies have shown that these changes in cell shape are powered by intracellular contractile networks comprising actin filaments, actin cross-linkers and myosin motors. In their Development at a Glance poster article, Munjal and Lecuit provide an overview of the mechanics, principles and regulation of actomyosin-driven cellular tension driving tissue morphogenesis. See the article on p. 1789
Bioengineering approaches to guide stem cell-based organogenesis
![]() Bioengineering approaches promise to bridge the gap between stem cell-driven tissue formation in culture and morphogenesis in vivo, offering mechanistic insight into organogenesis and unveiling powerful new models for drug discovery, as well as strategies for tissue regeneration in the clinic. Here, Lutolf and colleagues draw on several examples of stem cell-derived organoids to illustrate how bioengineering can contribute to tissue formation ex vivo. See the Review article on p. 1794
Bioengineering approaches promise to bridge the gap between stem cell-driven tissue formation in culture and morphogenesis in vivo, offering mechanistic insight into organogenesis and unveiling powerful new models for drug discovery, as well as strategies for tissue regeneration in the clinic. Here, Lutolf and colleagues draw on several examples of stem cell-derived organoids to illustrate how bioengineering can contribute to tissue formation ex vivo. See the Review article on p. 1794
Genomic imprinting in development, growth, behavior and stem cells
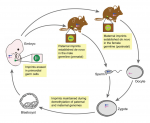 Genes that are subject to genomic imprinting in mammals are preferentially expressed from a single parental allele. These imprinted genes can directly regulate fetal growth, and recent work has also demonstrated intricate roles for imprinted genes in the brain and in induced pluripotent stem cells and adult stem cells. As Bartolomei and colleagues review here, these findings highlight the complex nature and developmental importance of imprinted genes. See the Review on p. 1805
Genes that are subject to genomic imprinting in mammals are preferentially expressed from a single parental allele. These imprinted genes can directly regulate fetal growth, and recent work has also demonstrated intricate roles for imprinted genes in the brain and in induced pluripotent stem cells and adult stem cells. As Bartolomei and colleagues review here, these findings highlight the complex nature and developmental importance of imprinted genes. See the Review on p. 1805


 (No Ratings Yet)
(No Ratings Yet)