In Development this week (Vol. 142, Issue 13)
Posted by Seema Grewal, on 30 June 2015
Here are the highlights from the current issue of Development:
Controlling Traffic jam for niche specification
 Tissue stem cells are dependent on their local microenvironment – the niche – for regulation of self-renewal and differentiation. In the Drosophila male germline, the niche comprises a set of 8-10 somatic cells called hub cells, which are located in a cluster at the anterior of the gonad. Specification of the niche is known to require Notch signalling and the transcription factor Bowl, but is not fully understood. Lindsey Wingert and Stephen DiNardo (p. 2268) now find that the Maf transcription factor Traffic jam (Tj) plays a key role in repressing hub cell development. In tj mutants, the number of somatic gonadal cells expressing hub markers increases. Tj is downregulated in hub cells in a Notch-dependent manner, and mutation of tj can rescue hub cell formation in Notch mutants. However, while Tj depletion can induce hub cell fate, it does not promote hub cell morphology or clustering. Instead, this requires Bowl activity – also probably acting downstream of Notch. Thus, Notch signalling regulates hub cell specification and hub assembly via different downstream effectors. Intriguingly, Maf factors are expressed in the mammalian gonad, where Notch also plays a role, raising the possibility that this mechanism is conserved.
Tissue stem cells are dependent on their local microenvironment – the niche – for regulation of self-renewal and differentiation. In the Drosophila male germline, the niche comprises a set of 8-10 somatic cells called hub cells, which are located in a cluster at the anterior of the gonad. Specification of the niche is known to require Notch signalling and the transcription factor Bowl, but is not fully understood. Lindsey Wingert and Stephen DiNardo (p. 2268) now find that the Maf transcription factor Traffic jam (Tj) plays a key role in repressing hub cell development. In tj mutants, the number of somatic gonadal cells expressing hub markers increases. Tj is downregulated in hub cells in a Notch-dependent manner, and mutation of tj can rescue hub cell formation in Notch mutants. However, while Tj depletion can induce hub cell fate, it does not promote hub cell morphology or clustering. Instead, this requires Bowl activity – also probably acting downstream of Notch. Thus, Notch signalling regulates hub cell specification and hub assembly via different downstream effectors. Intriguingly, Maf factors are expressed in the mammalian gonad, where Notch also plays a role, raising the possibility that this mechanism is conserved.
Cilia get sensitive with Notch
 In the vertebrate neural tube, dorso-ventral cell identity is regulated by Sonic Hedgehog (Shh) signalling: increasing pathway activity promotes progressively more ventral fates. Shh signal transduction involves primary cilia, where several of the key pathway components are localised. Now (p. 2291), Kim Dale and colleagues report a role for Notch signalling in regulating Shh activity in the neural tube. Through experiments in cell culture, chick explants and mouse embryos, the authors find that Notch modulates responsiveness to the Shh signal: activating the Notch pathway renders cells more sensitive to Shh. Notch appears to act by promoting ciliary localisation of the key Shh pathway component Smoothened, as well as increasing levels of the activator form of the transcriptional effector Gli3. Moreover, cilia are longer upon Notch pathway activation. Together, these data provide an unexpected connection between the Notch and Shh pathways in the vertebrate neural tube, which impacts on neuronal fate.
In the vertebrate neural tube, dorso-ventral cell identity is regulated by Sonic Hedgehog (Shh) signalling: increasing pathway activity promotes progressively more ventral fates. Shh signal transduction involves primary cilia, where several of the key pathway components are localised. Now (p. 2291), Kim Dale and colleagues report a role for Notch signalling in regulating Shh activity in the neural tube. Through experiments in cell culture, chick explants and mouse embryos, the authors find that Notch modulates responsiveness to the Shh signal: activating the Notch pathway renders cells more sensitive to Shh. Notch appears to act by promoting ciliary localisation of the key Shh pathway component Smoothened, as well as increasing levels of the activator form of the transcriptional effector Gli3. Moreover, cilia are longer upon Notch pathway activation. Together, these data provide an unexpected connection between the Notch and Shh pathways in the vertebrate neural tube, which impacts on neuronal fate.
Making furrows in the fly
 The early Drosophila embryo is a syncytium. Cellularisation only occurs after 14 very rapid nuclear divisions, the last four of which occur at the periphery and are associated with transient ingressions of the plasma membrane between dividing nuclei. How do these transient furrows form and what is their function? On p. 2316, Todd Blankenship and co-workers address these questions using live-imaging approaches. First, the authors characterise the dynamics of furrow formation and retraction. They then test whether the RalA GTPase, known to be important in other systems for targeted membrane addition via the exocyst complex, plays a role in furrow dynamics. Depletion of RalA leads to a lack of membrane ingression, and the authors also find that the Rab8 GTPase and the exocyst component Sec5 are important for ingression. In the absence of RalA, and hence furrows, two different types of nuclear division defects are seen: spindle anchoring is impaired, leading to chromosome mis-segregation at anaphase, and nuclei are not efficiently separated, leading to fusion of adjacent genomes. RalA-dependent transient furrow formation is therefore important to maintain genome integrity during the early syncytial divisions in Drosophila.
The early Drosophila embryo is a syncytium. Cellularisation only occurs after 14 very rapid nuclear divisions, the last four of which occur at the periphery and are associated with transient ingressions of the plasma membrane between dividing nuclei. How do these transient furrows form and what is their function? On p. 2316, Todd Blankenship and co-workers address these questions using live-imaging approaches. First, the authors characterise the dynamics of furrow formation and retraction. They then test whether the RalA GTPase, known to be important in other systems for targeted membrane addition via the exocyst complex, plays a role in furrow dynamics. Depletion of RalA leads to a lack of membrane ingression, and the authors also find that the Rab8 GTPase and the exocyst component Sec5 are important for ingression. In the absence of RalA, and hence furrows, two different types of nuclear division defects are seen: spindle anchoring is impaired, leading to chromosome mis-segregation at anaphase, and nuclei are not efficiently separated, leading to fusion of adjacent genomes. RalA-dependent transient furrow formation is therefore important to maintain genome integrity during the early syncytial divisions in Drosophila.
What leaders follow: FGF keeps duct on course
 Many organs contain tubular epithelia, and their development involves tubule elongation, lumen formation, and establishment and maintenance of tubular integrity. Yuji Atsuta and Yoshiko Takahashi (p. 2329) investigate how these processes are coordinated, using the chicken Wolffian (or nephric) duct (WD) as a model. The WD extends posteriorly from the pronephric region, between the presomitic mesoderm (PSM) and the lateral plate mesoderm. Cells at the front of the extending tissue show mesenchymal characteristics, while towards the rear, WD cells become more epithelial and the tubule lumen forms. Here, the authors find that WD migration is driven by a dynamic FGF gradient: FGF8 is expressed in the extending PSM and serves as a chemoattractant for the leading WD cells. Where FGF concentrations are low anteriorly in the segmented somites, the rear part of the WD epithelialises. Thus, as the body axis extends and the FGF source moves more posteriorly, the WD tube forms progressively, in a manner coordinated with whole-body elongation.
Many organs contain tubular epithelia, and their development involves tubule elongation, lumen formation, and establishment and maintenance of tubular integrity. Yuji Atsuta and Yoshiko Takahashi (p. 2329) investigate how these processes are coordinated, using the chicken Wolffian (or nephric) duct (WD) as a model. The WD extends posteriorly from the pronephric region, between the presomitic mesoderm (PSM) and the lateral plate mesoderm. Cells at the front of the extending tissue show mesenchymal characteristics, while towards the rear, WD cells become more epithelial and the tubule lumen forms. Here, the authors find that WD migration is driven by a dynamic FGF gradient: FGF8 is expressed in the extending PSM and serves as a chemoattractant for the leading WD cells. Where FGF concentrations are low anteriorly in the segmented somites, the rear part of the WD epithelialises. Thus, as the body axis extends and the FGF source moves more posteriorly, the WD tube forms progressively, in a manner coordinated with whole-body elongation.
Tissue interactions drive heart development
 During cardiac development, interactions between the endocardium – the endothelial lining of the heart – and the overlying myocardium are important for myocardial development, whereas BMP signals from the myocardium have been found to regulate late stages of endocardial morphogenesis. However, relatively little is known about early endocardial specification, or the potential role of the myocardium in this. Hedgehog signalling is known to be necessary but not sufficient for endocardial differentiation, but what other mechanisms are involved? Saulius Sumanas and colleagues now show (p. 2304) that myocardium-derived BMP plays a role in this early stage of endocardial development. When the myocardium is disrupted – either in hand2 mutants or upon genetic ablation of myocardial cells – endocardial fate fails to be specified or is lost in the endothelial progenitors. Expression of endocardial markers can be rescued by ectopic provision of BMP signals, while endocardial differentiation fails upon disruption of the BMP pathway. These data add a further layer of interaction between endocardium and myocardium, and underscore the importance of BMP signalling at multiple stages of heart development.
During cardiac development, interactions between the endocardium – the endothelial lining of the heart – and the overlying myocardium are important for myocardial development, whereas BMP signals from the myocardium have been found to regulate late stages of endocardial morphogenesis. However, relatively little is known about early endocardial specification, or the potential role of the myocardium in this. Hedgehog signalling is known to be necessary but not sufficient for endocardial differentiation, but what other mechanisms are involved? Saulius Sumanas and colleagues now show (p. 2304) that myocardium-derived BMP plays a role in this early stage of endocardial development. When the myocardium is disrupted – either in hand2 mutants or upon genetic ablation of myocardial cells – endocardial fate fails to be specified or is lost in the endothelial progenitors. Expression of endocardial markers can be rescued by ectopic provision of BMP signals, while endocardial differentiation fails upon disruption of the BMP pathway. These data add a further layer of interaction between endocardium and myocardium, and underscore the importance of BMP signalling at multiple stages of heart development.
PLUS…
An interview with Austin Smith
 Austin Smith is a stem cell and developmental biologist, who has dedicated his career to the study of pluripotency, stem cell renewal and differentiation. He is currently the Director of the Wellcome Trust MRC Cambridge Stem Cell Institute at the University of Cambridge, UK. We met him there to discuss his research and interests, as well as his role as an editor for Development. See the Spotlight article on p. 2227
Austin Smith is a stem cell and developmental biologist, who has dedicated his career to the study of pluripotency, stem cell renewal and differentiation. He is currently the Director of the Wellcome Trust MRC Cambridge Stem Cell Institute at the University of Cambridge, UK. We met him there to discuss his research and interests, as well as his role as an editor for Development. See the Spotlight article on p. 2227
LIF signaling in stem cells and development
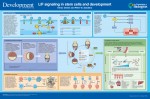 Leukemia inhibitory factor (LIF) is a member of the IL-6 cytokine family. All members of this family activate signal transducer and activator of transcription 3 (STAT3), a transcription factor that influences stem and progenitor cell identity, proliferation and cytoprotection. Here, Kento Onishi and Peter Zandstra provide an overview of JAK-STAT signaling during development. See the Development at a Glance article on p. 2230
Leukemia inhibitory factor (LIF) is a member of the IL-6 cytokine family. All members of this family activate signal transducer and activator of transcription 3 (STAT3), a transcription factor that influences stem and progenitor cell identity, proliferation and cytoprotection. Here, Kento Onishi and Peter Zandstra provide an overview of JAK-STAT signaling during development. See the Development at a Glance article on p. 2230
The never-ending story: from pluripotency to plant developmental plasticity
 Unlike most animals, plants employ a post-embryonic mode of development driven by the continuous activity of pluripotent stem cells. Consequently, plants are able to initiate new organs over extended periods of time, and many species can readily replace lost body structures by de novo organogenesis. Here, Christophe Gaillochet and Jan Lohmann review how pluripotency is established in plant stem cell systems, how it is maintained during development and growth and re-initiated during regeneration, and how these mechanisms eventually contribute to the amazing developmental plasticity of plants. See the Review on p. 2237
Unlike most animals, plants employ a post-embryonic mode of development driven by the continuous activity of pluripotent stem cells. Consequently, plants are able to initiate new organs over extended periods of time, and many species can readily replace lost body structures by de novo organogenesis. Here, Christophe Gaillochet and Jan Lohmann review how pluripotency is established in plant stem cell systems, how it is maintained during development and growth and re-initiated during regeneration, and how these mechanisms eventually contribute to the amazing developmental plasticity of plants. See the Review on p. 2237


 (No Ratings Yet)
(No Ratings Yet)