In Development this week (Vol. 142, Issue 17)
Posted by Seema Grewal, on 1 September 2015
Here are the highlights from the current issue of Development:
Tubulogenesis at single-cell resolution

Formation of the blood vessels involves complex endothelial morphogenesis, including directional cell migration and lumen formation. Understanding the cell biological processes underlying vessel formation in vivo requires the ability to analyse the behaviour of single cells at high spatiotemporal resolution within developing tissues, which has been challenging. Taking advantage of the genetic tools and optical clarity of zebrafish embryos, Brant Weinstein and colleagues (p.2951) develop a robust method to label both the nuclei and membranes of individual endothelial cells (ECs) and monitor their behaviour using two-photon imaging. Using these tools, they first investigate the cellular basis of the plexin D1knockdown phenotype. Their data suggest that the defective migration of ECs in plexin D1morphants can be attributed to differences in the protrusive activity of wild-type versus morphant ECs. In a second set of experiments, the authors show that lumens form both within and between ECs, suggesting that multiple mechanisms contribute to tubulogenesis in this system. In addition to providing insights into the cellular basis of vessel formation, this work presents a set of tools that should be valuable to the community for future analyses.
Oxygen helps the brain grow

It is known that adult neural stem cells are found in a hypoxic environment and are sensitive to oxygen tension. However, whether oxygen tension also regulates neural progenitor behaviour during development has been unclear. Now (p. 2904), Alexander Storch and co-workers investigate the effects of maternal hypoxia or hyperoxia on cortical development in mice. They find that exposure of pregnant mothers to different atmospheric oxygen tensions causes changes in foetal brain oxygenation, and that this has consequences on overall brain volume: hypoxic embryos have smaller brains, whereas hyperoxia causes increased brain size. At the cellular level, the hypoxic foetal cortex shows reduced proliferation of progenitors and increased apoptosis. By contrast, hyperoxia induces the accumulation of a population of proliferative progenitor cells in regions of the cortex more basal to the main zones of proliferation. These cells, similar to the outer subventricular zone progenitors that are normally rare in rodents but more common in primates, apparently contribute to increased corticogenesis. Although the mechanisms by which oxygen signalling regulates cortical development remain unknown, these intriguing results point to an important role for oxygen tension during foetal neurogenesis.
RAy regeneration: one pathway, many roles
The zebrafish caudal fin is a powerful model for understanding the cellular and molecular processes underlying regeneration. Following amputation, a proliferative blastema forms, from which all the tissues of the fin regrow. Many aspects of this regenerative process are still poorly understood. For example, what is the source of the various lineages, how do these diverse cell types differentiate in appropriate proportions, and how is their spatial patterning controlled? In two related papers, Nicola Blum and Gerrit Begemann investigate the regeneration of the fin rays, uncovering crucial roles for retinoic acid (RA) signalling – which is known to be important for bone formation during development – at multiple steps of the process.

On p. 2894), the authors uncover a complex series of requirements for active or suppressed RA pathway activity in the bone lineage. RA is known to be synthesized in the blastema immediately following amputation, but the authors find that this inhibits dedifferentiation of osteoblasts to a proliferative preosteoblast state. Subsequently, RA signalling promotes proliferation of the preosteoblasts, then inhibits their differentiation, and is finally required for new bone matrix production. Given these apparently opposing roles for RA at different stages of ray regeneration, levels of the RA-synthesizing enzyme Aldh1a2 and the RA-degrading enzyme Cyp26b1 appear to be tightly regulated in the blastema, in both a spatial and temporal manner.

On p. 2888), Blum and Begemann analyse the role of RA signalling in the spatial control of ray regeneration, such that each ray is separated by an interray region. They find that Cyp26a1 (a paralogue of the blastema-expressed Cyp26b1 discussed above) is expressed in the basal epidermal layer overlying the osteoblasts but absent from the cells overlying the interray tissue. Thus, a low-RA environment exists in the epidermis around the regenerating ray. This permits expression of Sonic hedgehog (Shh), again specifically in the epidermis around the ray, which in turn promotes proliferation of osteoblasts. When this spatial restriction of RA is abolished – using Cyp26a1 inhibitors – osteoblasts spread into the interray regions and the patterning of the regenerating fin is disrupted. Together, these two studies reveal multiple roles for RA in ray regeneration, and highlight the complex signalling dynamics required to achieve efficient and precise regeneration.
Hippo/Notch cross-talk in the neural crest

Vascular smooth muscle is derived, in part, from the neural crest in a differentiation programme regulated by the Jagged1-Notch signalling pathway. However, there is also evidence that Hippo signalling regulates vascular smooth muscle development. Several studies have detailed mechanisms by which the Hippo and Notch pathways interact and, on p. 2962, Jonathan Epstein and colleagues identify a new level of cross-talk between these two pathways – via direct interaction between Yap (a Hippo pathway-regulated transcription factor) and the Notch intracellular domain (NICD). They first show in mice that deletion of Yap and the functionally related protein Taz in the neural crest leads to loss of crest-derived vascular smooth muscle; this phenotype is reminiscent of that caused by deletion of Rbp-J – the transcription factor downstream of Notch. They then provide evidence in cell culture models that Yap and NICD physically interact and co-occupy the enhancers of a subset of known Notch target genes, and that the Yap/NICD/Rbp-J complex is important for induction of vascular smooth muscle differentiation. Thus, as well as shedding light on how the vascular smooth muscle forms, this work provides insights into the mechanisms of signalling pathway cross-talk and its importance during development.
PLUS:
An interview with Didier Stainier
 Didier Stainier is a Principal Investigator at the Max Planck Institute for Heart and Lung Research in Bad Nauheim, Germany. Having spent most of his career in the USA using zebrafish to study organ development, Didier recently moved back to Europe and is now branching out to study organ development in mice. At a recent conference, we caught up with Didier and asked him about his career, his thoughts on funding, view on morpholinos and his advice for young researchers. See the Spotlight on p. 2861
Didier Stainier is a Principal Investigator at the Max Planck Institute for Heart and Lung Research in Bad Nauheim, Germany. Having spent most of his career in the USA using zebrafish to study organ development, Didier recently moved back to Europe and is now branching out to study organ development in mice. At a recent conference, we caught up with Didier and asked him about his career, his thoughts on funding, view on morpholinos and his advice for young researchers. See the Spotlight on p. 2861
Neuromesodermal progenitors and the making of the spinal cord
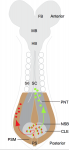 Neuromesodermal progenitors (NMps) contribute to both the elongating spinal cord and the adjacent paraxial mesoderm. It has been assumed that these cells arise as a result of anterior neural plate patterning. However, as the molecular mechanisms that specify NMps in vivo are uncovered, and as protocols for generating these bipotent cells from mouse and human pluripotent stem cells in vitro are established, the emerging data suggest that this view needs to be revised. See the Hypothesis by Storey et al on p. 2864
Neuromesodermal progenitors (NMps) contribute to both the elongating spinal cord and the adjacent paraxial mesoderm. It has been assumed that these cells arise as a result of anterior neural plate patterning. However, as the molecular mechanisms that specify NMps in vivo are uncovered, and as protocols for generating these bipotent cells from mouse and human pluripotent stem cells in vitro are established, the emerging data suggest that this view needs to be revised. See the Hypothesis by Storey et al on p. 2864
Trithorax and Polycomb group-dependent regulation: a tale of opposing activities
 Polycomb and Trithorax group chromatin proteins play important roles promoting the stable and heritable repression and activation of gene expression, respectively. Here, Geisler and Paro review recent advances that have shed light on the mechanisms by which these two classes of proteins maintain epigenetic memory and allow dynamic switches in gene expression during development. See the Review on p. 2876
Polycomb and Trithorax group chromatin proteins play important roles promoting the stable and heritable repression and activation of gene expression, respectively. Here, Geisler and Paro review recent advances that have shed light on the mechanisms by which these two classes of proteins maintain epigenetic memory and allow dynamic switches in gene expression during development. See the Review on p. 2876
Featured movie
Our latest featured movie shows ocular morphogenesis in a zebrafish embryo. It is from a recent paper where Link and colleagues show that the transcriptional regulators Yap/Taz and Tead are necessary and sufficient for optic vesicle progenitors to adopt retinal pigmented epithelium identity. Read the paper here: http://bit.ly/1KJIPqP [OPEN ACCESS]


 (No Ratings Yet)
(No Ratings Yet)