In Development this week (Vol. 142, Issue 4)
Posted by Seema Grewal, on 10 February 2015
Here are the highlights from the current issue of Development:
Pathways to human hypothalamic neurons
 The dysfunction of hypothalamic neurons is implicated in a number of common diseases, including obesity, hypertension, and mood and sleep disorders. To date, studies of human hypothalamic neurons have been limited due to their inaccessibility, but now (on p. 633), Florian Merkle, Kevin Eggan, Alex Schier and colleagues use two complimentary techniques to differentiate human pluripotent stem cells (hPSCs) into an array of neuropeptide-producing hypothalamic neurons. In the first approach, the researchers use a self-patterning strategy to generate hypothalamic progenitors from hPSCs (both embryonic stem cells and induced pluripotent stem cells). The subsequent maturation of these progenitors leads to the generation of neuropeptide-producing neurons that are morphologically similar to their in vivo counterparts. In the second, more readily scalable approach, the researchers direct the differentiation of hPSCs into hypothalamic progenitors by modulating pathways known to play a role in hypothalamic development. These progenitors, they report, can also be matured into neuropeptidergic neurons that adopt the gene expression profiles and morphological properties of their counterparts in the human brain. Finally, the authors demonstrate that in vitro-derived human hypothalamic neurons are able to integrate into the mouse brain and continue to express hypothalamus-specific neuropeptides. The cells generated by these approaches thus offer a promising tool for disease modelling, drug screening and therapeutic cell transplantation.
The dysfunction of hypothalamic neurons is implicated in a number of common diseases, including obesity, hypertension, and mood and sleep disorders. To date, studies of human hypothalamic neurons have been limited due to their inaccessibility, but now (on p. 633), Florian Merkle, Kevin Eggan, Alex Schier and colleagues use two complimentary techniques to differentiate human pluripotent stem cells (hPSCs) into an array of neuropeptide-producing hypothalamic neurons. In the first approach, the researchers use a self-patterning strategy to generate hypothalamic progenitors from hPSCs (both embryonic stem cells and induced pluripotent stem cells). The subsequent maturation of these progenitors leads to the generation of neuropeptide-producing neurons that are morphologically similar to their in vivo counterparts. In the second, more readily scalable approach, the researchers direct the differentiation of hPSCs into hypothalamic progenitors by modulating pathways known to play a role in hypothalamic development. These progenitors, they report, can also be matured into neuropeptidergic neurons that adopt the gene expression profiles and morphological properties of their counterparts in the human brain. Finally, the authors demonstrate that in vitro-derived human hypothalamic neurons are able to integrate into the mouse brain and continue to express hypothalamus-specific neuropeptides. The cells generated by these approaches thus offer a promising tool for disease modelling, drug screening and therapeutic cell transplantation.Plant embryogenesis: the ins and outs of auxin flow
 Directional transport of the plant hormone auxin plays an essential role in plant development. To date, most studies of auxin transport have focussed on the PIN family of auxin efflux transporters but now Jiri Friml and colleagues show that the AUX1 and LIKE-AUX1 (LAX) auxin influx carriers are required during plant embryogenesis (p. 702). The researchers first demonstrate that the pharmacological inhibition of auxin influx in both microspore-derived Brassica napus embryos and Arabidopsis thaliana embryos results in defects in early embryogenesis. They further reveal thatAUX1, LAX1 and LAX2, but not LAX3, are expressed during Arabidopsis embryo development. These differentially expressed influx carriers, they report, are required for correct embryo development; patterning defects and defective cotyledon and root formation are observed inaux1 lax1 lax2 triple mutants. Further genetic interaction studies reveal that aux/lax and pinmutations have additive effects on cotyledon development, suggesting that AUX/LAX carriers act in concert with PIN transporters. Finally, the researchers uncover a positive-feedback loop between MONOPTEROS-dependent auxin signalling and auxin transport, highlighting a role for balanced and regulated auxin influx and efflux during plant development.
Directional transport of the plant hormone auxin plays an essential role in plant development. To date, most studies of auxin transport have focussed on the PIN family of auxin efflux transporters but now Jiri Friml and colleagues show that the AUX1 and LIKE-AUX1 (LAX) auxin influx carriers are required during plant embryogenesis (p. 702). The researchers first demonstrate that the pharmacological inhibition of auxin influx in both microspore-derived Brassica napus embryos and Arabidopsis thaliana embryos results in defects in early embryogenesis. They further reveal thatAUX1, LAX1 and LAX2, but not LAX3, are expressed during Arabidopsis embryo development. These differentially expressed influx carriers, they report, are required for correct embryo development; patterning defects and defective cotyledon and root formation are observed inaux1 lax1 lax2 triple mutants. Further genetic interaction studies reveal that aux/lax and pinmutations have additive effects on cotyledon development, suggesting that AUX/LAX carriers act in concert with PIN transporters. Finally, the researchers uncover a positive-feedback loop between MONOPTEROS-dependent auxin signalling and auxin transport, highlighting a role for balanced and regulated auxin influx and efflux during plant development.Morphogenesis in full force
 Convergent extension (CE) is a morphogenetic process that shapes the early vertebrate embryo. During CE, embryonic tissues elongate along one axis while narrowing in the other, but how are the appropriate forces generated and regulated during this event? Here, Lance Davidson and colleagues investigate the mechanical control of CE in Xenopus embryos (p. 692). They develop a new method for measuring tissue-scale force production, which involves embedding embryonic tissues in a gel-based force sensor. Using this approach, they report that force production during CE is regulated by myosin II contractility; reduced elongation rates are seen when tissues are treated with a ROCK inhibitor but, surprisingly, these are only evident when tissues are challenged with the mechanical constraints of the gel. By altering gel composition, the researchers further demonstrate that CE is adaptive and can accommodate to stiffer microenvironments, suggesting that mechanical feedback produces greater stresses to overcome the constraints of the gel. Finally, they report that the notochord does not contribute to force production, whereas the paraxial mesoderm and prospective neural tissues are major contributors to elongation forces. This study thus sheds light on the mechanical control of CE and offers an exciting new tool that can be used to probe force production in developing tissues.
Convergent extension (CE) is a morphogenetic process that shapes the early vertebrate embryo. During CE, embryonic tissues elongate along one axis while narrowing in the other, but how are the appropriate forces generated and regulated during this event? Here, Lance Davidson and colleagues investigate the mechanical control of CE in Xenopus embryos (p. 692). They develop a new method for measuring tissue-scale force production, which involves embedding embryonic tissues in a gel-based force sensor. Using this approach, they report that force production during CE is regulated by myosin II contractility; reduced elongation rates are seen when tissues are treated with a ROCK inhibitor but, surprisingly, these are only evident when tissues are challenged with the mechanical constraints of the gel. By altering gel composition, the researchers further demonstrate that CE is adaptive and can accommodate to stiffer microenvironments, suggesting that mechanical feedback produces greater stresses to overcome the constraints of the gel. Finally, they report that the notochord does not contribute to force production, whereas the paraxial mesoderm and prospective neural tissues are major contributors to elongation forces. This study thus sheds light on the mechanical control of CE and offers an exciting new tool that can be used to probe force production in developing tissues.Lending weight to mitochondrial transmission
 Over-nutrition and obesity during pregnancy are known to result in lasting developmental and metabolic consequences in offspring. Here, using the Blobby mouse model for obesity, Rebecca Robker and co-workers (p. 681) probe the mechanistic origins of these changes and test if they are reversible. They demonstrate that obese females produce cumulus oocyte complexes that exhibit changes in gene and protein expression associated with ER stress. They further show that oocytes from obese mice contain normal levels of mitochondrial (mt) DNA, but display reduced mitochondrial membrane potential and higher levels of autophagy compared with control oocytes. Following in vitro fertilization, the oocytes from obese mice form blastocysts that contain reduced levels of mtDNA and exhibit reduced developmental potential. When transferred to normal-weight surrogates, these blastocysts give rise to foetuses that are heavier than controls and exhibit reduced mtDNA content in the kidney and liver. Importantly, many of these obesity-dependent changes in oocyte characteristics and developmental potential can be reversed by treatment with ER stress inhibitors. Together, these studies demonstrate that obesity induces mitochondrial dysfunction that is transmitted to offspring, and that these defects can be alleviated by using interventions prior to conception to improve embryo and foetal development.
Over-nutrition and obesity during pregnancy are known to result in lasting developmental and metabolic consequences in offspring. Here, using the Blobby mouse model for obesity, Rebecca Robker and co-workers (p. 681) probe the mechanistic origins of these changes and test if they are reversible. They demonstrate that obese females produce cumulus oocyte complexes that exhibit changes in gene and protein expression associated with ER stress. They further show that oocytes from obese mice contain normal levels of mitochondrial (mt) DNA, but display reduced mitochondrial membrane potential and higher levels of autophagy compared with control oocytes. Following in vitro fertilization, the oocytes from obese mice form blastocysts that contain reduced levels of mtDNA and exhibit reduced developmental potential. When transferred to normal-weight surrogates, these blastocysts give rise to foetuses that are heavier than controls and exhibit reduced mtDNA content in the kidney and liver. Importantly, many of these obesity-dependent changes in oocyte characteristics and developmental potential can be reversed by treatment with ER stress inhibitors. Together, these studies demonstrate that obesity induces mitochondrial dysfunction that is transmitted to offspring, and that these defects can be alleviated by using interventions prior to conception to improve embryo and foetal development.Vascular patterning goes out on a limb
 During limb morphogenesis, developing digits are initially interconnected by soft tissue but then become separated as this tissue undergoes programmed cell death (PCD) and regresses. Now, Elazar Zelzer and co-workers demonstrate that vascular patterning in the mouse limb regulates interdigital cell death by a reactive oxygen species (ROS)-dependent mechanism (p. 672). They demonstrate the presence of a complex and high-density capillary network within interdigital regions at the onset of PCD; by contrast, the developing digits are unvascularized. As PCD progresses, they report, the vasculature concomitantly becomes remodelled. They further show that a decrease in interdigital blood vessel numbers, induced by inactivating VEGF in the limb mesenchyme, inhibits PCD. By contrast, hypervascularization following VEGF overexpression in the limb leads to elevated PCD and an expansion of the domain in which PCD occurs. Finally, the researchers demonstrate that interdigital PCD is dependent on oxygen and the production of ROS. Together, these findings highlight a novel function for vascular patterning, and suggest the existence of a mechanism by which vascularization of interdigital regions leads to an increase in tissue oxygenation, which in turn triggers ROS production and PCD.
During limb morphogenesis, developing digits are initially interconnected by soft tissue but then become separated as this tissue undergoes programmed cell death (PCD) and regresses. Now, Elazar Zelzer and co-workers demonstrate that vascular patterning in the mouse limb regulates interdigital cell death by a reactive oxygen species (ROS)-dependent mechanism (p. 672). They demonstrate the presence of a complex and high-density capillary network within interdigital regions at the onset of PCD; by contrast, the developing digits are unvascularized. As PCD progresses, they report, the vasculature concomitantly becomes remodelled. They further show that a decrease in interdigital blood vessel numbers, induced by inactivating VEGF in the limb mesenchyme, inhibits PCD. By contrast, hypervascularization following VEGF overexpression in the limb leads to elevated PCD and an expansion of the domain in which PCD occurs. Finally, the researchers demonstrate that interdigital PCD is dependent on oxygen and the production of ROS. Together, these findings highlight a novel function for vascular patterning, and suggest the existence of a mechanism by which vascularization of interdigital regions leads to an increase in tissue oxygenation, which in turn triggers ROS production and PCD.Activin/Nodal signalling in stem cells
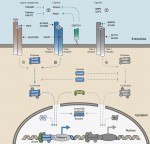 Activin/Nodal growth factors control a broad range of biological processes, including early cell fate decisions, organogenesis and adult tissue homeostasis. Here, Siim Pauklin and Ludovic Vallier provide an overview of the mechanisms by which the Activin/Nodal signalling pathway governs stem cell function in these different stages of development. See the Review on p. 607
Activin/Nodal growth factors control a broad range of biological processes, including early cell fate decisions, organogenesis and adult tissue homeostasis. Here, Siim Pauklin and Ludovic Vallier provide an overview of the mechanisms by which the Activin/Nodal signalling pathway governs stem cell function in these different stages of development. See the Review on p. 607
The melanocyte lineage in development and disease
 Melanocytes have an apparently simple aetiology, differentiating from the neural crest and migrating through the developing embryo to specific locations within the skin and hair follicles, and to other sites in the body. Here, using a cross-species approach, Richard Mort, Ian Jackson and Elizabeth Patton discuss melanocyte development and differentiation, melanocyte stem cells, and the role of the melanocyte lineage in diseases such as melanoma.See the Review on p. 620
Melanocytes have an apparently simple aetiology, differentiating from the neural crest and migrating through the developing embryo to specific locations within the skin and hair follicles, and to other sites in the body. Here, using a cross-species approach, Richard Mort, Ian Jackson and Elizabeth Patton discuss melanocyte development and differentiation, melanocyte stem cells, and the role of the melanocyte lineage in diseases such as melanoma.See the Review on p. 620


 (No Ratings Yet)
(No Ratings Yet)