In Development this week (Vol. 142, Issue 7)
Posted by Seema Grewal, on 24 March 2015
Here are the highlights from the current issue of Development:
ActivinA-ting spiny neuron production from hPSCs
 The medium-sized spiny neurons, the main projection neurons of the striatum, are generated in the lateral ganglionic eminence (LGE) and degenerate in the early stages of Huntington’s disease (HD) – for which no pharmacological treatment is yet available. Hence, an efficient way to derive striatal neurons is crucial for disease modelling, drug development and cell-replacement therapy. Striatal neurons have previously been generated from human pluripotent stem cell (hPSC)-derived neural progenitors treated with sonic hedgehog (SHH), or SHH plus Wnt pathway inhibition. Now, Meng Li and co-workers (p. 1375) report a more robust and efficient way to generate functional striatal neurons from hPSCs. They show that activin A induces LGE characteristics in hPSC-derived neural progenitors. This is independent of SHH but requires CTIP2, a transcription factor required for striatal neuron development. Furthermore, the activin-patterned neural precursors efficiently generate functional DARPP32+ GABAergic striatal neurons in vitro, and acquire striatal spiny neuron properties without overgrowth or teratoma formation upon engraftment in a rat HD model. Altogether, these findings uncover a novel role for activin A in striatal projection neuron specification and establish a robust protocol for deriving these neurons.
The medium-sized spiny neurons, the main projection neurons of the striatum, are generated in the lateral ganglionic eminence (LGE) and degenerate in the early stages of Huntington’s disease (HD) – for which no pharmacological treatment is yet available. Hence, an efficient way to derive striatal neurons is crucial for disease modelling, drug development and cell-replacement therapy. Striatal neurons have previously been generated from human pluripotent stem cell (hPSC)-derived neural progenitors treated with sonic hedgehog (SHH), or SHH plus Wnt pathway inhibition. Now, Meng Li and co-workers (p. 1375) report a more robust and efficient way to generate functional striatal neurons from hPSCs. They show that activin A induces LGE characteristics in hPSC-derived neural progenitors. This is independent of SHH but requires CTIP2, a transcription factor required for striatal neuron development. Furthermore, the activin-patterned neural precursors efficiently generate functional DARPP32+ GABAergic striatal neurons in vitro, and acquire striatal spiny neuron properties without overgrowth or teratoma formation upon engraftment in a rat HD model. Altogether, these findings uncover a novel role for activin A in striatal projection neuron specification and establish a robust protocol for deriving these neurons.
Cofilin the gap in neural tube closure
 Neural tube closure occurs through highly orchestrated cell shape changes mediated by actin dynamics. Its failure results in some of the most common and severe human congenital malformations. Cofilin 1, an actin-depolymerising protein, is known to be involved in neural tube closure but its precise functions had not been elucidated. In this study (p. 1305), Joaquim Grego-Bessa and colleagues show that the absence of cofilin 1 in mouse leads to defective neural tube closure, reduced cell number, altered cell shape and cell cycle kinetics. The protein is enriched at both apical and basal domains of the neuroepithelium but, intriguingly, has opposing activities on either side of the cell. Apically, although localisation of the apical polarity complexes remains unchanged, phosphorylation of myosin light chain is impaired in cofilin 1 mutants. By contrast, basally, the absence of cofilin 1 leads to an accumulation of actin and phosphorylated myosin light chain, ectopic tight junction-like structures and disruption of the basement membrane and hence of epithelial organisation. Altogether, these results shed light on the cellular mechanisms of neural tube closure and reveal a dual role for cofilin that is presumably dependent on the intracellular context.
Neural tube closure occurs through highly orchestrated cell shape changes mediated by actin dynamics. Its failure results in some of the most common and severe human congenital malformations. Cofilin 1, an actin-depolymerising protein, is known to be involved in neural tube closure but its precise functions had not been elucidated. In this study (p. 1305), Joaquim Grego-Bessa and colleagues show that the absence of cofilin 1 in mouse leads to defective neural tube closure, reduced cell number, altered cell shape and cell cycle kinetics. The protein is enriched at both apical and basal domains of the neuroepithelium but, intriguingly, has opposing activities on either side of the cell. Apically, although localisation of the apical polarity complexes remains unchanged, phosphorylation of myosin light chain is impaired in cofilin 1 mutants. By contrast, basally, the absence of cofilin 1 leads to an accumulation of actin and phosphorylated myosin light chain, ectopic tight junction-like structures and disruption of the basement membrane and hence of epithelial organisation. Altogether, these results shed light on the cellular mechanisms of neural tube closure and reveal a dual role for cofilin that is presumably dependent on the intracellular context.
Preserving progenitor pools in the kidney: a balancing act
The nephrons are the filtration units of the kidney that excrete toxins, balance salt and water content in the blood and regulate blood pressure. Their number is determined during kidney development by the size of the nephron progenitor cell (NPC) pool, which exhausts in early postnatal life in mouse. Understanding the mechanisms that regulate the balance between NPC self-renewal and differentiation is a crucial endeavour. In this issue, two papers provide insights into the molecular cues controlling NPC self-renewal.
 On p. 1228, Zubaida Saifudeen and colleagues report that the specific deletion of p53 in mouse NPCs leads to hypoplastic kidneys, reduced nephron number and elevated blood pressure. p53 is classically associated with restraining proliferation, but the observed phenotype suggests a positive role for p53 in progenitor renewal: in mutants, NPC proliferation is reduced while senescence, apoptosis and the levels of known regulators of NPC survival remain unchanged. Furthermore, using functional genomics, the authors find that p53 regulates factors involved in cell-matrix interactions and metabolism. They then show that mutants display aberrant ATP and reactive oxygen species levels in NPCs. Altogether, these results uncover an unexpected contribution of p53 to NPC self-renewal capacity, energy metabolism and niche architecture.
On p. 1228, Zubaida Saifudeen and colleagues report that the specific deletion of p53 in mouse NPCs leads to hypoplastic kidneys, reduced nephron number and elevated blood pressure. p53 is classically associated with restraining proliferation, but the observed phenotype suggests a positive role for p53 in progenitor renewal: in mutants, NPC proliferation is reduced while senescence, apoptosis and the levels of known regulators of NPC survival remain unchanged. Furthermore, using functional genomics, the authors find that p53 regulates factors involved in cell-matrix interactions and metabolism. They then show that mutants display aberrant ATP and reactive oxygen species levels in NPCs. Altogether, these results uncover an unexpected contribution of p53 to NPC self-renewal capacity, energy metabolism and niche architecture. In the second study (p. 1254), Martin Kann and co-workers identify growth arrest-specific 1 (Gas1) as a direct target of Wilms’ tumor suppressor protein 1 (WT1), a transcription factor required for NPC self-renewal and differentiation. Phenotypically, the loss of GAS1 is similar to p53 depletion, with mutant mice displaying hypoplastic kidneys and decreased nephron numbers, stemming from reduced NPC proliferation. The authors further analyse the mechanism by which GAS1 acts in NPCs, finding that it modulates the response to fibroblast growth factor (FGF) signalling, a known regulator of NPC growth and proliferation, by specifically promoting the AKT pathway branch downstream of receptor activation. This study therefore links WT1 to FGF-mediated regulation of NPC proliferation, providing additional insights into the mechanisms by which this key transcription factor functions.
In the second study (p. 1254), Martin Kann and co-workers identify growth arrest-specific 1 (Gas1) as a direct target of Wilms’ tumor suppressor protein 1 (WT1), a transcription factor required for NPC self-renewal and differentiation. Phenotypically, the loss of GAS1 is similar to p53 depletion, with mutant mice displaying hypoplastic kidneys and decreased nephron numbers, stemming from reduced NPC proliferation. The authors further analyse the mechanism by which GAS1 acts in NPCs, finding that it modulates the response to fibroblast growth factor (FGF) signalling, a known regulator of NPC growth and proliferation, by specifically promoting the AKT pathway branch downstream of receptor activation. This study therefore links WT1 to FGF-mediated regulation of NPC proliferation, providing additional insights into the mechanisms by which this key transcription factor functions.
PLUS…
Positional information and reaction-diffusion: two big ideas in developmental biology combine
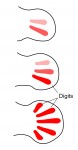 The two most influential ideas in the field of pattern formation are those of Alan Turing’s ‘reaction-diffusion’ and Lewis Wolpert’s ‘positional information’. Much has been written about these two concepts but some confusion still remains, in particular about the relationship between them. Here, Jeremy Green and James Sharpe address this relationship and propose a scheme of three distinct ways in which these two ideas work together to shape biological form. See their Hypothesis article on p. 1203
The two most influential ideas in the field of pattern formation are those of Alan Turing’s ‘reaction-diffusion’ and Lewis Wolpert’s ‘positional information’. Much has been written about these two concepts but some confusion still remains, in particular about the relationship between them. Here, Jeremy Green and James Sharpe address this relationship and propose a scheme of three distinct ways in which these two ideas work together to shape biological form. See their Hypothesis article on p. 1203
Cellular and molecular insights into Hox protein action
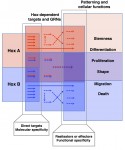 Hox genes encode homeodomain transcription factors that control morphogenesis and have established functions in development and evolution.Here, Yacine Graba and colleagues discuss the molecular and cellular mechanisms underlying the diverse and context-dependent functions of Hox transcription factors during morphogenesis and organogenesis. See the Review article on p. 1212
Hox genes encode homeodomain transcription factors that control morphogenesis and have established functions in development and evolution.Here, Yacine Graba and colleagues discuss the molecular and cellular mechanisms underlying the diverse and context-dependent functions of Hox transcription factors during morphogenesis and organogenesis. See the Review article on p. 1212


 (1 votes)
(1 votes)