Investigating the Impact of Oligodendrocyte Precursor Cell Ablation in Mouse Models of Growth Hormone Deficiency
Posted by Sonja Lutz, on 5 October 2023
This summer, I had the opportunity to work at the Francis Crick Institute. Because my early university experience was disrupted by COVID, receiving an opportunity to get some real research and lab experience was something I could not pass up. The Crick has a very collaborative and diverse work environment, along with a range of weekly interest groups and seminars; therefore, coming to the Crick was an easy decision to make. I worked in the group of Robin Lovell-Badge and I was supervised and mentored by Richard Clayton who is a postdoc in the lab.
Oligodendrocyte precursor cells and the median eminence of the hypothalamus
Robin’s lab focuses on the genetics of development and stem cells; Richard’s work in the lab is to investigate the role of oligodendrocyte lineage cells in health and disease. Oligodendrocyte precursor cells (OPCs) are glial cells of the central nervous system that have some stem cell-like properties. OPCs eventually differentiate into myelinating oligodendrocytes, but also into other cell types to a lesser extent (Akay, Effenberger and Tsai, 2021). While I was at the Crick, I got involved in studying the OPCs of the median eminence (ME), which is a small section of the hypothalamus. The ME contains nerve endings of neurons that control the secretion of pituitary hormones and thus, the function of the hypothalamic-pituitary axes and neuroendocrine system is dependent on the ME (Clayton, Lovell-Badge and Galichet, 2022). Proper functioning of the neuroendocrine system is vital for healthy bodily functions.
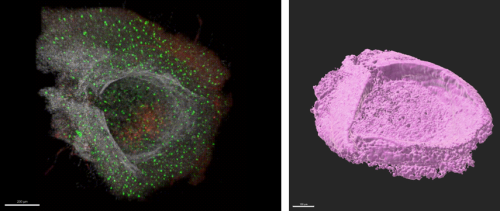
Figure 1: Confocal projection of a dissected median eminence (left) and the same data as a 3D render(right). The green cells are microglia, in red are OPCs, and the white channel is nuclei.
Figure 1 shows one of the MEs I got to dissect from a mouse brain. The images show that the ME is like a little boat or cup shape which sits right at the base of the hypothalamus. Where the pituitary gland attaches to the pituitary stalk can be seen in the image on the left. This dissection was extremely difficult as the ME is tiny, and could only be seen due to a few key blood vessels that can be seen under a stereo microscope. In one of the genetically modified mice used in the lab, the OPCs are marked with red fluorescence (Galichet, Clayton and Lovell-Badge, 2021); the image shows a high density of them at the bottom of the ‘boat’ that is the ME (Figure 1).
OPCs may be involved in regulation of the neuroendocrine system from within the ME, for example, one study found that OPCs in this region are important for body weight control and leptin sensing (Djogo et al., 2016). Another study from within my lab has found deficits in proliferation and differentiation of OPCs in the ME in mouse models that have hypopituitarism – specifically, mice that are mutant for certain Sox genes (Galichet, Rizzoti and Lovell-Badge, 2023). Cells which were previously thought to simply function as precursors to oligodendrocytes may actually play a much bigger role. An open question is whether OPCs in this part of the brain could control growth by regulating growth hormone (GH) secretion.
The hypothalamus, growth hormone, and side-effects of radiotherapy
Post-natal growth is driven by the hypothalamic-pituitary-somatotropic axis. Figure 2 shows how GH is released from the pituitary. The somatotropic axis involves the release of growth hormone releasing hormone (GHRH) from the arcuate nucleus of the hypothalamus. This then travels down through the ME into the anterior pituitary. Here, somatotrophs release GH into the blood stream for a variety of functions, including the release of insulin-like growth factor-1 (IGF-1), which is vital for post-natal growth.
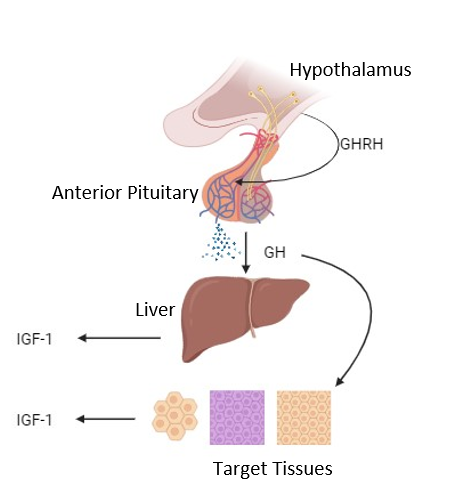
Figure 2: The hypothalamic-pituitary-somatotropic axis. Created with BioRender.com
Changes in GH levels can result in hypopituitarism, or growth hormone deficiency (GHD), leading to a range of diseases and symptoms, including a lack of normal growth. Approximately 50% of children that undergo cranial radiotherapy will develop a neuroendocrine disorder like GHD (Merchant et al., 2011). Since radiotherapy works by targeting rapidly dividing cells, and because OPCs are the most proliferative cell type in the brain, hypopituitarism has been linked with a decrease in OPC differentiation and survival. Therefore, one of the aims of my project was to characterize the changes of OPC numbers and hormone levels in mouse models of GHD.
Mouse model of GHD caused by radiation
One of these models involves the use of X-ray radiation to the brain to achieve the OPC ablation. However, radio-ablation of OPCs is inherently hard to study, as they are very re-generative and proliferative. Once ablated, new OPCs return rapidly. GHD is also difficult to measure due to the pulsatile nature of GH secretion (levels in the blood are known to fluctuate over time).
Nonetheless, we hypothesized that radiation would ablate the OPCs and result in hypopituitarism. Importantly, the chances of developing GHD increase if patients are younger at the time of the radiotherapy treatment (Pollock and Cohen, 2021). Hence, part of the project also aims to establish whether irradiation affects GH levels and OPCs differently at different ages. I counted the numbers of OPCs and measured the amount of GH and myelin levels in mice that were irradiated at a young age, and compared this to mice that were irradiated as adults.

Figure 3: This graph shows the levels of growth hormone (GH) between sham control and irradiated male mice. The asterisk represents statistical significance with a p-value <0.05.
Figure 3 illustrates results from the ELISA I carried out on the younger mouse cohort; it shows that there is a significant decrease in GH levels of irradiated male mice. This indicates to us that these mice are a potential model for GHD.
Correlating with OPC numbers: Immunostaining and imaging
Next, I carried out a range of immuno-stains for certain cellular proteins on coronal mouse brain sections to correlate the changes in GH with changes in OPCs in the brain. These proteins include myelin basic protein (Mbp), which stains for myelin; Olig2, which stains oligodendrocytes and their precursor cells; Pdgfrα, which stains OPCs, and DAPI, which stains DNA and therefore helps visualize cell nuclei.
Prior to staining, the mice would be culled and I would dissect the brain. I then processed the brains by sectioning them into smaller sections. I’d either cut it with the vibratome or the cryostat. The vibratome slices by pushing its blade across the sample at high vibrational frequencies; this was for sections around 50um thick. The cryostat was for sections around 10um thick, and used a fine blade kept at around -20C. Both were difficult to get used to at first, but I got better over the 9 weeks. The process was very exciting and I really enjoyed being able to personally visualise the anatomy.
Once sliced, I’d carry out the immuno-stains for different marker proteins and then mount the slides for imaging. I visualized the slides under the confocal microscope.
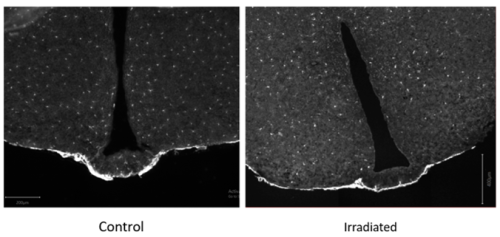
Figure 4: This shows two coronal sections of mouse brains, focused on the median eminence and the ventral hypothalamus. The left shows the control sham, and the right the irradiated. The sections are stained for Pdgfra+ in white, which is a marker of OPCs and also the meninges.
Figure 4 indicates that there is a clear reduction in OPCs in the ME of adult irradiated mouse brains. It also appears that there may be less within the arcuate nucleus of the hypothalamus, as well as the cortex. A surprising find was that within irradiated mice, there are parts of the brain that are less affected by the radiation – mainly the thalamus. This confirmed a previous observation of a difference in sensitivity of different regions of the brain to radiation (Irvine and Blakemore, 2007).
Perhaps the OPCs in the cortex and the ME are simply much more proliferative than those in the thalamus and hypothalamus. Another idea is that the OPCs in differing brain regions have different origins, functions, and properties that may make them more resistant to irradiation. This was quite an exciting idea, because if some OPCs are intrinsically resistant to radiation, we could use their properties to design a therapy that could subsequently make the OPCs of the ME resistant to radiation. Perhaps this could aid us with respect to the radiotherapy-linked GHD problem.
A genetic mouse model of growth deficiency
Next, I wanted to understand more about how loss of OPCs could lead to GHD. One idea is that OPCs are needed to support development and maintenance of GHRH-secreting neurons. To investigate, I used another mouse model where mice have mutant copies of a Sox gene. These mice have a growth deficit phenotype that indicates they could have GHD. The mice I used also had a fluorescent marker present in GHRH-secreting neurons, which meant I could count the overall number of neurons in the brains of these animals.
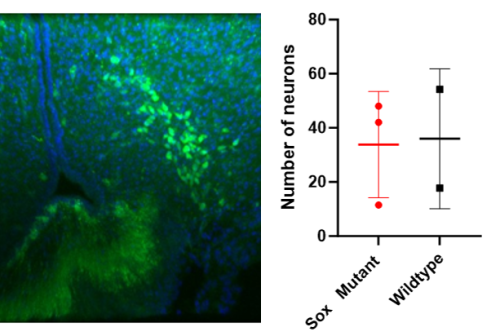
Figure 5: This shows the arcuate hypothalamus under the confocal microscope (left) stained for DAPI (blue) and GHRH neurons (green). The graph on the right compares the average GHRH neuron counts of the arcuate hypothalamus between the Sox mutant and wildtype mice.
The preliminary results suggest there is no difference in the number of GHRH neurons of Sox mutant vs wildtype mice (Figure 5). This suggests that the lack of GH is not due to a relative reduction in neurons in this model. Therefore, it may imply that while the number of neurons is unchanged, the Sox mutation affects the OPCs in a way that leads to a decrease in the amount of GHRH released. We could investigate this further by staining for recently active GHRH-secreting neurons to investigate their activity, both in Sox mutants but also in irradiated mice. Furthermore, we could carry out an ELISA or radioimmunoassay for GHRH to directly investigate the hormone levels.
Overall, my summer at the Crick was an unforgettable experience; I wish I could do it all over again. I learned an incredible amount over the 9 weeks, not just from my lab, but from the many seminars and talks that were available to us, and from all of my fellow summer students. Being in an environment where I was encouraged to always ask questions, and being somewhere where the scientific conversations continued over lunch and coffee, were some of my favourite aspects of my time at the Crick. The lab I was in had incredibly passionate and smart people in it, who made sure weekly lab meetings and coffee breaks were never boring. I thoroughly enjoyed being around people who share the same interests as me and who always want to learn.
I also gained valuable experience in conducting research and gained new skills of wet- and dry-lab techniques. I am a lot more confident and excited for my final year of undergraduate study, and I feel much more prepared – mentally and physically – for my dissertation project. Lastly, this experience has also opened the new avenue of pursuing a PhD which, until now, I had not properly considered.
Finally, I would like to thank the Francis Crick Institute for hosting me and the Medical Research Foundation Rosa Beddington Fund for supporting my project. I would also like to say thank you to everyone I met at the Crick. I had such a great experience, and I fully recommend that everyone should do a summer studentship!
https://www.crick.ac.uk/research/labs/robin-lovell-badge
REFERENCES
Akay, L.A., Effenberger, A.H. and Tsai, L.-H. (2021) ‘Cell of all trades: oligodendrocyte precursor cells in synaptic, vascular, and immune function’, Genes & Development, 35(3–4), pp. 180–198. Available at: https://doi.org/10.1101/gad.344218.120.
Clayton, R.W., Lovell-Badge, R. and Galichet, C. (2022) ‘The Properties and Functions of Glial Cell Types of the Hypothalamic Median Eminence’, Frontiers in Endocrinology, 13, p. 953995. Available at: https://doi.org/10.3389/fendo.2022.953995.
Djogo, T. et al. (2016) ‘Adult NG2-Glia Are Required for Median Eminence-Mediated Leptin Sensing and Body Weight Control’, Cell Metabolism, 23(5), pp. 797–810. Available at: https://doi.org/10.1016/j.cmet.2016.04.013.
Galichet, C., Clayton, R.W. and Lovell-Badge, R. (2021) ‘Novel Tools and Investigative Approaches for the Study of Oligodendrocyte Precursor Cells (NG2-Glia) in CNS Development and Disease’, Frontiers in Cellular Neuroscience, 15, p. 673132. Available at: https://doi.org/10.3389/fncel.2021.673132.
Galichet, C., Rizzoti, K. and Lovell-Badge, R. (2023) ‘Sox3-null hypopituitarism depends on median eminence NG2-glia and is influenced by aspirin and gut microbiota’. bioRxiv, p. 2023.07.26.550616. Available at: https://doi.org/10.1101/2023.07.26.550616.
Irvine, K.-A. and Blakemore, W.F. (2007) ‘A different regional response by mouse oligodendrocyte progenitor cells (OPCs) to high-dose X-irradiation has consequences for repopulating OPC-depleted normal tissue’, European Journal of Neuroscience, 25(2), pp. 417–424. Available at: https://doi.org/10.1111/j.1460-9568.2007.05313.x.
Merchant, T.E. et al. (2011) ‘Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors’, Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 29(36), pp. 4776–4780. Available at: https://doi.org/10.1200/JCO.2011.37.9453.
Pollock, N.I. and Cohen, L.E. (2021) ‘Growth Hormone Deficiency and Treatment in Childhood Cancer Survivors’, Frontiers in Endocrinology, 12, p. 745932. Available at: https://doi.org/10.3389/fendo.2021.745932.


 (4 votes)
(4 votes)