Just because it looks like a duct, doesn’t mean it is the duct
Posted by Janel Kopp, on 25 January 2011
The Node’s staff has kindly given me the opportunity to write a background piece, placing into context the results of our studies described in the paper, “Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas” (released today in Development; http://dev.biologists.org/lookup/doi/10.1242/dev.056499).
For many years, debate has raged in the pancreas biology field as to the source of new insulin-producing beta cells in the adult pancreas, both in healthy and injured states. This is a topic of great interest as many groups around the world are engaged in the quest to repopulate functional beta cell mass in diabetic patients, either through transplantation of hESC/iPSC-derived beta cells, or by stimulating growth of residual beta cells in such patients. Manipulating endogenous pathways of beta cell regeneration, should they exist, might prove to be one avenue of curing diabetes. Thus, this is a hot-button topic.
In 2007, when I joined Dr. Maike Sander’s laboratory, the diabetes field was heatedly pursuing the question of whether or not new beta cells can arise from pancreatic ducts. The possibility that ducts might harbor facultative progenitor cells capable of producing beta cells upon stimulation energized many labs to do experiments to test this theory. All of this attention was mainly due to studies that observed beta cells closely juxtaposed to pancreatic ducts after pancreatic injury (Gu et al., 1994). This was complemented by exciting data showing that Ngn3, an endocrine progenitor marker, is re-expressed in the ductal epithelium of beta cell regenerative models, such as partial duct ligation (PDL) (Xu et al., 2008). Yet another study, published by Rovira and colleagues, demonstrated that cells at the very end of the ductal tree (centroacinar/terminal duct cells) could be isolated from mice and behave like progenitors cells in the dish as well as differentiate correctly in an embryonic environment (Rovira et al., 2010). These findings all pointed to pancreatic ductal cells as a source of new pancreatic beta cells. That is until groups started to create and test CreER mouse lines with expression specifically in ductal cells (Furuyama et al., 2010; Kopinke et al., 2011; Kopinke and Murtaugh, 2010; Means et al., 2008; Solar et al., 2009) in the hopes of tracing duct-derived beta cells. I am a part of one of those groups.
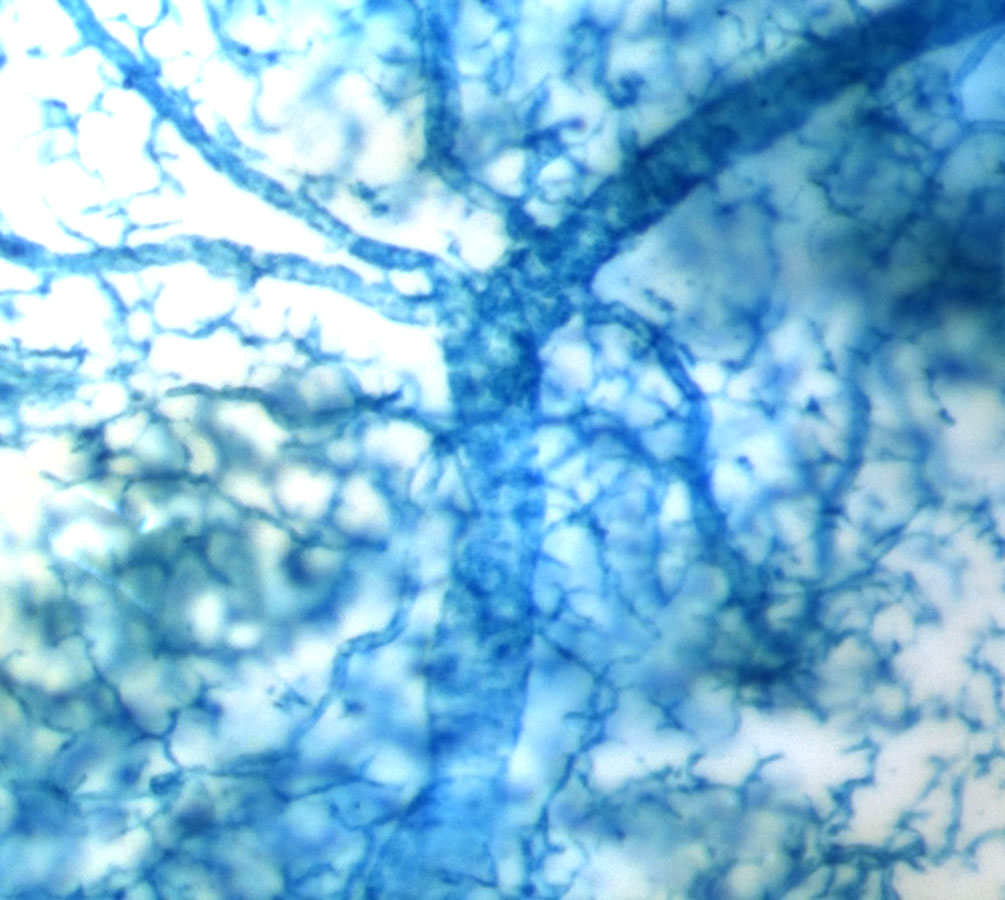
Sox9creER labeled pancreatic ductal tree
In our group, we created a Sox9-driven CreERT2 BAC transgenic mouse line and were thrilled to find that we could efficiently and exclusively label the pancreatic ductal tree in the adult Sox9CreERT2;R26RLacZ mouse (show picture). Given that the Sox9CreERT2 transgene labeled such a large percentage of ductal cells (~70%), we felt confident that if any beta cells arose from the ductal compartment after PDL, we would be the group to detect it. Therefore, I teamed up with Claire Dubois, a graduate student in Dr. Sander’s laboratory, to perform PDL on tamoxifen-injected Sox9CreERT2;R26RYFP mice. As predicted by Xu et al. (2008), we observed a large increase in Ngn3 mRNA in the ligated pancreatic lobe and a low signal for Ngn3 expression was found in duct-like foci derived from Sox9+ ductal cells after PDL. Much to our surprise though, PDL did not induce the production of new beta cells from lineage-labeled ductal cells. This suggests that Ngn3 expression is initiated in Sox9+ cells after PDL, but the presence of Ngn3 is not sufficient to initiate endocrine differentiation. Therefore our findings and the majority other studies published thus far do not support the hypothesis that adult pancreatic ductal cells contribute to the endocrine compartment during normal aging or after PDL.
Because many studies, including our study published today, agreed that acinar cells are maintained by self-replication and are not produced by other cell types (Desai et al., 2007; Jensen et al., 2005), I had focused on the question of endocrine neogenesis in the pancreas. However, Furuyama and colleagues recently created a knock-in Sox9IRES-CreERT2 mouse line and showed that Sox9+ cells can produce acinar, but not endocrine cells, in the adult mouse (Furuyama et al., 2010). How do we explain the discrepancy between their findings and ours? While we don’t fully understand the reason, small, but possibly significant, differences in the experimental design could provide an explanation. The tamoxifen doses used by Furuyama and colleagues were extremely high and resulted in labeling of acinar cells upon tamoxifen administration. Likewise, we observed patchy acinar cell labeling with our highest dosage of tamoxifen. It is possible that acinar cells express Sox9 at low levels, but recombination only occurs when the concentration of tamoxifen reaches a certain threshold. However, with the tamoxifen dosages used in our study the percentage of labeled acinar cells did not increase during the chase period. As it is unclear how long CreER remains active after very high dosages of tamoxifen, it is possible that rather than arising from Sox9+ ductal cells, in Furuyama’s study acinar cells are continuously labeled for an extended period of time after the tamoxifen pulse. Thus, additional studies showing results similar to those of Furuyama et al. will be necessary before it can be concluded that ductal cells contribute widely to the production of acinar cells.
Does this mean that ductal cells are not capable of producing other pancreatic cell types? The ability of ductal cells to form endocrine and acinar cells during development and the ex vivo analysis of terminal duct/centroacinar cells (Rovira et al., 2010) would suggest that ductal cells can be multipotent under the right circumstances. Therefore, future comparisons of the embryonic and adult ducts, as well as their microenvironments, may provide the key to turning a duct cell into an acinar or beta cell.
Janel L. Kopp, Claire L. Dubois, Ashleigh E. Schaffer, Ergeng Hao, Hung Ping Shih, Philip A. Seymour, Jenny Ma, & Maike Sander (2011). Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas Development, 138 (4), 653-665 : 10.1242/dev.056499
Desai, B. M., Oliver-Krasinski, J., De Leon, D. D., Farzad, C., Hong, N., Leach, S. D. and Stoffers, D. A. (2007). Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 117, 971-7.
Furuyama, K., Kawaguchi, Y., Akiyama, H., Horiguchi, M., Kodama, S., Kuhara, T., Hosokawa, S., Elbahrawy, A., Soeda, T., Koizumi, M. et al. (2010). Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43, 34-41.
Gu, D., Lee, M. S., Krahl, T. and Sarvetnick, N. (1994). Transitional cells in the regenerating pancreas. Development 120, 1873-81.
Jensen, J. N., Cameron, E., Garay, M. V., Starkey, T. W., Gianani, R. and Jensen, J. (2005). Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128, 728-41.
Kopinke, D., Brailsford, M., Shea, J. E., Leavitt, R., Scaife, C. L. and Murtaugh, L. C. (2011). Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development 138, 431-41.
Kopinke, D. and Murtaugh, L. C. (2010). Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol 10, 38.
Means, A. L., Xu, Y., Zhao, A., Ray, K. C. and Gu, G. (2008). A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis 46, 318-23.
Rovira, M., Scott, S.-G., Liss, A. S., Jensen, J., Thayer, S. P. and Leach, S. D. (2010). Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proceedings of the National Academy of Sciences 107, 75-80.
Solar, M., Cardalda, C., Houbracken, I., Martín, M., Maestro, M. A., De Medts, N., Xu, X., Grau, V., Heimberg, H. and Bouwens, L. (2009). Pancreatic Exocrine Duct Cells Give Rise to Insulin-Producing β Cells during Embryogenesis but Not after Birth. Developmental Cell 17, 849-860.
Xu, X., D’Hoker, J., Stange, G., Bonne, S., De Leu, N., Xiao, X., Van de Casteele, M., Mellitzer, G., Ling, Z., Pipeleers, D. et al. (2008). Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197-207.


 (8 votes)
(8 votes)