Making Some Noise About Morphogens
Posted by UChicagoDRSB_JC, on 1 November 2013
Greetings, Node readers! We at The University of Chicago have just resumed our yearly Development, Regeneration and Stem Cell Biology Journal Club. I would like to take this opportunity to thank this year’s student organizer, Steve Briscoe. Steve is a 3rd year student in the DRSB program and a member of Dr. Cliff Ragsdale’s laboratory. He is already doing an excellent job arranging speakers and making sure refreshments are provided (keeping both students and faculty happy). Thanks, Steve! This month’s post comes from our very first meeting, in which we discussed Xiong et al.’s recent paper Specified Neural Progenitors Sort to Form Sharp Domains after Noisy SHH Signaling (Cell. Vol. 153, Issue 3, 25 April 2013, Pages 550-561).
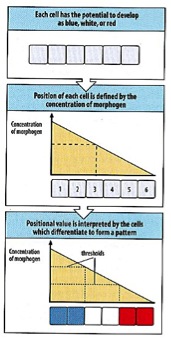
Morphogens hold a special place in the heart of developmental biologists. Hated by some, frustrating many, attracting countless others, morphogens have captivated scholars for many years. The beauty of the morphogen lies in its ability to create complex patterns, which often have both structural and functional significance. These patterns frequently appear as sharp delineations of cell types in a developing tissue.
The classic model of how such specific patterns can form is described in Lewis Wolpert’s “French flag” model (1969). Imagine a gradient of a diffusible morphogen across a field of unspecified cells, drawn as large blank rectangles (fig. 1). As the morphogen diffuses from source to sink, the concentration of the signal drops, creating a gradient from high to low concentration. Within this gradient, there are threshold concentrations to which cells can respond. The fate of each cell is determined by its position in the signaling field; that is, which threshold concentration the cell encounters. A cell receiving a high concentration of the morphogen will respond differently from a cell receiving a low concentration of the morphogen, creating a spatial pattern, such as the three broad stripes of the French flag (fig. 1).
However, to create the sharp boundaries of cell types seen in embryos, this model relies on precise signal responses at stable cell locations. But, as we all know, development is a messy business. Cells in developing tissues are not typically sitting still; rather, they undergo complex movement, migration, division, and death. How can a clear pattern come from this chaos? Xiong et al. (2013) provide a possible answer as to how sharp boundaries of cell types form in a dynamic, growing tissue.
An excellent example of such a tissue can be found in the vertebral neural tube, in which sharply defined progenitor domains form along the dorsal-ventral axis. It is thought that cells in the neural tube respond to a ventral-to-dorsal gradient of Sonic Hedgehog (SHH), entering a specific state of gene expression relative to the SHH levels encountered. At this point, intracellular gene regulatory network interactions between SHH-related transcription factors establish discrete cell fates, which are no longer dependent on SHH signaling (Xiong et al. 2013 and reverences therein). Again, however, we are faced with an important question. How can cells receive such precise spatial and temporal cues when they are moving and proliferating?
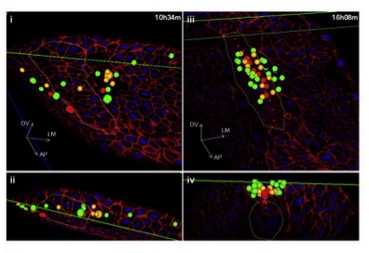
Using a new imaging platform they term “in toto imaging” of zebrafish, Xiong et al. investigated the neural tube in more detail. They not only analyzed the pattern of cell specification, but also investigated the migration trajectories of the neural tube progenitors. Instead of the expected “French flag-style” separation of specified progenitor cells, the researchers discovered that cells with different fates were spatially mixed in the developing neural tube (fig. 2). Thus, there seem to be heterogeneous signaling responses to SHH in the neural tube. The authors also show that the cells are sorted out into discrete domains based on their fate (fig 2), although how this task is accomplished is yet unknown.
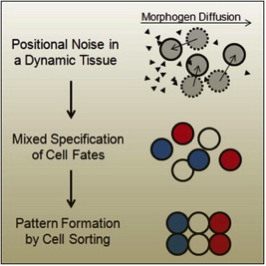
Overall, it seems that cell sorting acts to correct the imprecision of a gradient-system with noisy inductive signals in a dynamic tissue (fig. 3). Many have pondered the morphogen gradient, wondering how such a system could really function in the embryonic milieu. With this study, we have another way to consider the way in which precise, beautiful and functional patterns are enacted in dynamic tissues. Exciting future work in other classic morphogen-gradient systems will determine whether this is an isolated case or a widely-spread phenomenon.
This post was composed by Haley K. Stinnett, PhD Candidate in the department of Organismal Biology and Anatomy at the University of Chicago.


 (6 votes)
(6 votes)