Orphan nuclear receptors: individual and collective roles in mouse development
Posted by Nicola_Festuccia, on 10 February 2025
The elusive importance of NR5A2 and ESRRB as pluripotency factors
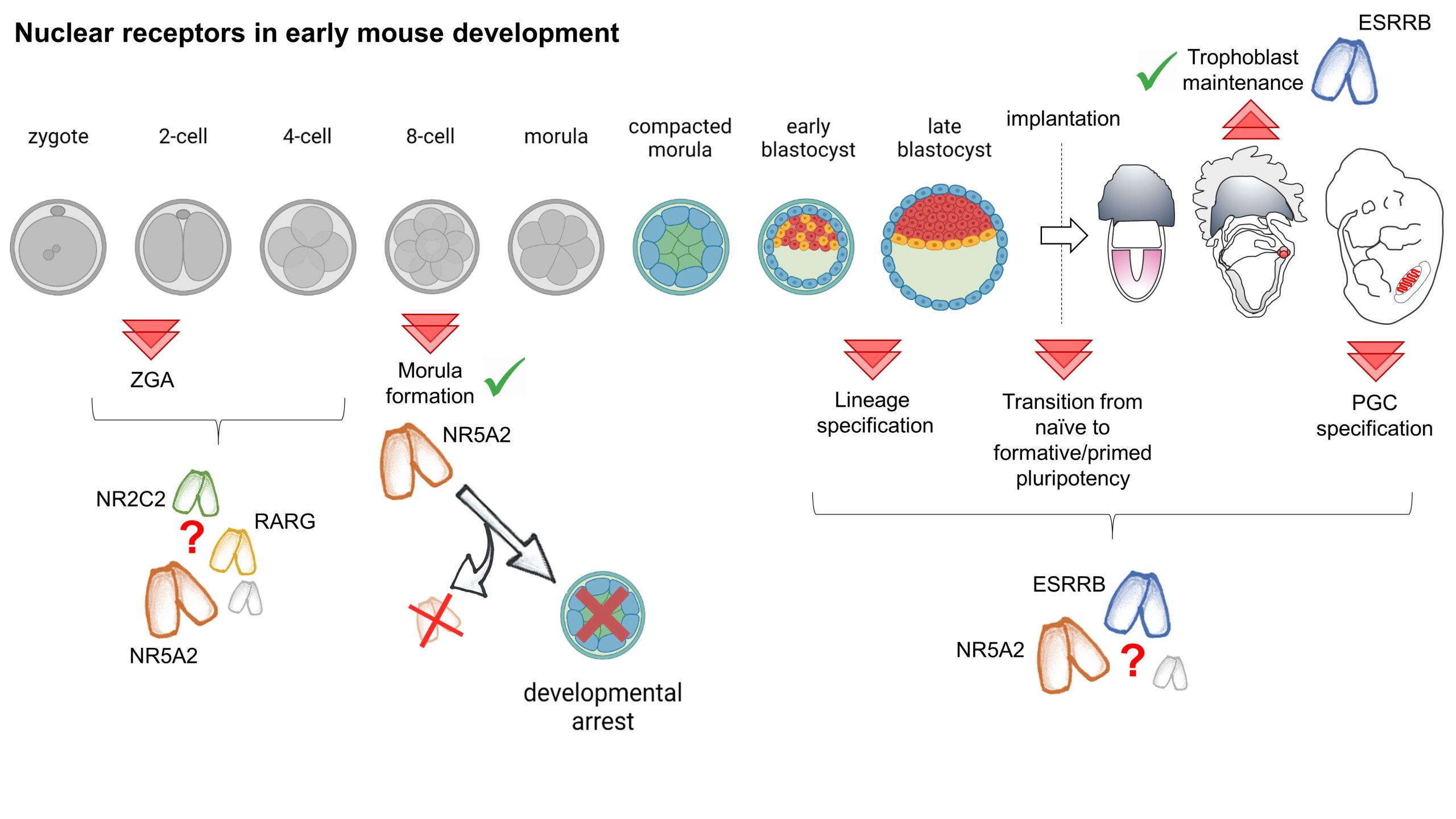
Our paper entitled “Nr5a2 is dispensable for zygotic genome activation but essential for morula development” is the culmination of a long scientific journey working on mouse embryonic stem cells (ESCs), which I initiated during my PhD in the group of Ian Chambers, in Edinburgh, where we showed that the nuclear receptor Esrrb acts as a pluripotency transcription factor (TF) [1]. Back then, we also first asked the question that eventually led to our recent publication: if ESRRB is important for pluripotency, how can embryos specify an epiblast in the absence of this TF? In fact, the available literature showed that Esrrb KO embryos do not manifest evident defects in the pluripotent lineage [2, 3]. This contradiction became more strident in the following years. My work, first as a postdoc and then as a permanent CNRS researcher in the group of Pablo Navarro in Paris, together with results from several other groups, showed that ESRRB performs unique functions in ESCs: it conveys external signals[4, 5], binding and maintaining the activity of key nodes of the pluripotency network[6], including during mitosis [7-9]. As a consequence, the loss of ESRRB marks the beginning of differentiation and, conversely, ESRRB expression drives the completion of somatic cell reprograming [1, 10-12].
We soon came to realise that the role of ESRRB in pluripotent cells cannot be understood without considering its interaction with other nuclear receptors, especially NR5A2. Indeed, while ES cells tolerate the individual loss of either ESRRB or NR5A2, in the simultaneous absence of these two nuclear receptors the pluripotency network collapses remarkably fast [9, 13]. Thus, while remaining distinct TFs, ESRRB and NR5A2 act as a single functional unit, which rivals in importance with that of the core components of the pluripotency network: their redundant activity is strictly required for pluripotency.
Shared and unique roles of NR5A2 in building a morula
Having identified two essential players in the maintenance of ES cell identity, we now had the knowledge needed to test the role that these two orphan nuclear receptors play in driving the establishment of pluripotency in vivo. We hypothesized that the lack of a phenotype in Esrrb KO embryos was likely due to a compensation by NR5A2. To test this, we joined efforts with the team led by Michel Cohen Tannoudji, who had joined Pablo’s group. Together, we generated animals carrying floxed alleles for both genes and expressing Zp3:Cre, which drives recombination in growing oocytes. We crossed these females with heterozygous males, and recovered control, single KO, and double KO embryos at the blastocyst stage. This experiment, a few years in the making, generated the promised exciting results… but unceremoniously contradicted our expectations! While controls had almost completed lineage specification, Nr5a2 KO embryos appeared severely degenerated, and this is… irrespective of Esrrb expression [14].
Following on this unexpected observation, our work, together with the results of two independent reports from the groups of Wei Xie [15] and Kikue Tachibana [16], revealed an essential developmental role for Nr5a2. Rapidly upregulated after the activation of transcription from the zygotic genome (ZGA), which occurs at the 2-cell stage (2C-stage) in mouse, NR5A2 controls the first wave of embryonic gene expression. NR5A2 targets include many key developmental regulators, such as Nanog, Klf5, Sox15, Tead4, Gata3 and Cdx2, but also genes supporting housekeeping functions, such as cell division, and DNA repair. As a consequence, during the two rounds of cell divisions that follow the activation of transcription, NR5A2 performs a dual function: it lays the ground for the beginning of lineage specification, all while maintaining genomic stability. While the contribution of ESRRB appears minor, NR5A2 does not act alone during this developmental window. At least two other TFs expressed just after ZGA, KLF5 [17, 18] and TCFP2AC [19], have been shown to contribute in preparing the segregation of the first two embryonic lineages, endowing blastomeres with the potential to generate both trophectoderm and the inner cell mass (ICM). NR5A2 activates Tcfp2a and Klf5 expression, and seems to act in conjunction with Krüppel-like factors to establish chromatin accessibility in 8C-stage embryos [14]. Reciprocally, TCFP2A activates Nr5a2 [19]. Therefore, an interconnected regulatory network, characteristic of the 8C-stage, and governed by the interaction between regulators of extraembryonic and embryonic fates, is open to future research.
The role of NR5A2 in maintaining genome stability might, instead, be specific to this TF. After the 8-cell stage, Nr5a2 KO embryos start displaying cell division defects, which progressively exacerbate, and eventually arrest before forming a blastocyst. Besides activating expression of genes that are required for the execution of cell division, NR5A2 might execute functions that are directly linked to its activity as a bookmarking TF. Is the ability of orphan nuclear receptors to remain bound to specific genomic region during mitosis playing an unforeseen role in ensuring the faithful partitioning of the genetic material? Irrespectively, despite opening a number of novel questions, these results establish that NR5A2 is required for the formation of a developmentally competent and viable morula.
Orphan nuclear receptors and the first wave of gene expression
While the transcriptional effect of the KO of Nr5a2 manifest fully only at the 8C-stage, the earlier role of this TF remains less clear. Treating embryos with chemical inhibitors targeting NR5A2, among other nuclear receptors, or depleting NR5A2 protein in ex-vivo experiments has been shown to disrupt ZGA and cause developmental arrest starting at the 2C-stage [16]. A role as an initiator of ZGA implies a determinant function of maternally inherited NR5A2. However, our experiments show that females producing oocytes devoid of Nr5a2 can give birth to live pups, and the Zp3:Cre-driven maternal or maternal and zygotic KO of Nr5a2 affects only a few hundred genes at the late 2C-stage, and compromises the activation of a minority of the genes that start being transcribed during ZGA. Similarly mild effects have been reported after the siRNA-mediated knockdown of Nr5a2 [15, 16]. In addition, we showed that the developmental defects of Nr5a2 KO embryos can be rescued by injecting Nr5a2 mRNA in 4C-stage embryos. Conversely, triggering the loss of Nr5a2 after ZGA, by the use of an inducible Cre driver, mimics the phenotype of the maternal and zygotic KO. What is the base of the discrepancy between different studies? Extending our original hypothesis, it is possible to envisage functional interactions with yet other nuclear receptors expressed at this stage of development. Among these, two stand out: NR2C2, which is also sensitive to NR5A2 inhibitors [16], and the retinoic acid receptor RARG, which has been shown to play a role in 2C embryos, and in driving ZGA [20]. Both nuclear receptors present an expression profile analogous to Nr5a2 in early embryos. In addition, among the few genes downregulated by the KO of Nr5a2 at the onset of ZGA we found a number of know regulators of the 2C transcriptional programme, including Zscan4 genes, themselves involved in activating embryonic transcription. Curiously, while NR5A2 seems to activate these genes at the time of ZGA, at later stages mutant embryo fail, to a variable extent, to correctly repress their expression. Therefore, the interplay between different nuclear receptors and other classes of TFs in controlling the 2C-stage transcriptional programme, ZGA, and the progression past this stage of development remain an active area of investigation. Despite these uncertainties, it is however clear that NR5A2 accumulates in the blastomeres as early as the late 2C stage; if not playing a role in initiating ZGA, this TF surely starts regulating embryonic gene expression soon after the onset of embryonic transcription.
Open Questions
Despite the excitement brought by these results, our work leaves our original question standing. New tools need to be developed to bypass the requirement for NR5A2 in the formation of a morula able to begin lineage specification, and assess the conjunct role that ESRRB and NR5A2 may play during the execution of these cell fate choices. Indeed, beside an essential role as a pluripotency TFs, and potentially in the establishment of pluripotency, experiments in ESCs indicate that ESRRB and NR5A2 might have both overlapping and distinct functions in the segregation of the epiblast from the primitive endoderm [21-24].
Looking beyond pre-implantation development, ESRRB and NR5A2 might also play a role in germline specification. ESRRB expression is extinguished in the epiblast at implantation [3, 14]. Studies in ESCs indicate that, before being downregulated, ESRRB may prime the transition between the gene regulatory networks that support pre- and post-implantation pluripotency [25]. These changes are in turn required for the activation of the germ cell programme in a few cells in the posterior region of the epiblast. Both Esrrb and Nr5a2, together with a number of other pluripotency regulators, are then re-expressed in these nascent primordial germ cells [26]. In-vivo and in-vitro studies hinted at the involvement of the two orphan receptors in multiple aspect of germ cell specification [3, 25, 27] but did not report an essential function in supporting germ cell identity. However, functional compensation might have masked the role of orphan receptors also in this context.
Developmental TF: lone wolves or social animals?
Our work illustrates both the individual and collective importance of orphan nuclear receptors in regulating cell identity and developmental processes. These TFs, able to access the very same sites on DNA, show sign of a functional redundancy that goes beyond the generic cooperativity between TFs [13]. However, if ESRRB and NR5A2 are collectively required for the maintenance of pluripotency in ESCs, and possibly the epiblast, we have observed that NR5A2 alone is essential for morula development. Could then redundancy be inbuilt in the control of some developmental processes and not others? And if so with what implications? Redundancy provides a fail-safe mechanism to ensure the robustness of crucial gene regulatory processes, and has been observed in other TF families active during early development such as Krüppel-like and Gata factors [17, 28, 29]. Studies ranging from the analysis of stress response TF in yeast [30] to work on retinoic acid receptors [31], indicate that redundant TF pairs, while showing the ability to regulate a substantial fraction of common target genes, often maintain some degree of specificity. This property can be exploited to achieve robust expression of a common set of genes, while retaining the ability to modulate either of the uniquely regulated gene sets. One could imagine that some of the genes exclusively regulated by a TF operating redundantly with NR5A2, for instance ESRRB, must not be activated or repressed during morula formation, when NR5A2 expression peaks, and its effects are dominant. Ensuring strong activation of a common gene set, like that supporting the pluripotency programme, might instead be later required during epiblast specification, when NR5A2 levels are declining and ESRRB is still robustly expressed. With potential functions spanning through events that link fertilisation to the establishment of the germline, acting across cell cycle phases, and in a collective or individual fashion, studying nuclear receptors promises to teach us much about how redundancy contributes to the making and unmaking cell identity during development. Are developmental TFs lone wolves or social animals?
References
1. Festuccia, N., et al., Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell, 2012. 11(4): p. 477-90.
2. Luo, J., et al., Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature, 1997. 388(6644): p. 778-82.
3. Mitsunaga, K., et al., Loss of PGC-specific expression of the orphan nuclear receptor ERR-beta results in reduction of germ cell number in mouse embryos. Mech Dev, 2004. 121(3): p. 237-46.
4. Martello, G., et al., Esrrb is a pivotal target of the gsk3/tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell, 2012. 11(4): p. 491-504.
5. Hamilton, W.B., et al., Dynamic lineage priming is driven via direct enhancer regulation by ERK. Nature, 2019. 575(7782): p. 355-360.
6. Whyte, W.A., et al., Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell, 2013. 153(2): p. 307-19.
7. Festuccia, N., et al., Mitotic binding of Esrrb marks key regulatory regions of the pluripotency network. Nat Cell Biol, 2016. 18(11): p. 1139-1148.
8. Festuccia, N., et al., Transcription factor activity and nucleosome organization in mitosis. Genome Res, 2019. 29(2): p. 250-260.
9. Chervova, A., et al., Mitotic bookmarking redundancy by nuclear receptors in pluripotent cells. Nat Struct Mol Biol, 2024.
10. Huang, D., et al., LIF-activated Jak signaling determines Esrrb expression during late-stage reprogramming. Biol Open, 2018. 7(1).
11. Buganim, Y., et al., Single-Cell Expression Analyses during Cellular Reprogramming Reveal an Early Stochastic and a Late Hierarchic Phase. Cell, 2012. 150(6): p. 1209-22.
12. Polo, J.M., et al., A molecular roadmap of reprogramming somatic cells into iPS cells. Cell, 2012. 151(7): p. 1617-32.
13. Festuccia, N., et al., The combined action of Esrrb and Nr5a2 is essential for murine naive pluripotency. Development, 2021. 148(17).
14. Festuccia, N., et al., Nr5a2 is dispensable for zygotic genome activation but essential for morula development. Science, 2024. 386(6717): p. eadg7325.
15. Lai, F., et al., NR5A2 connects zygotic genome activation to the first lineage segregation in totipotent embryos. Cell Res, 2023. 33(12): p. 952-966.
16. Gassler, J., et al., Zygotic genome activation by the totipotency pioneer factor Nr5a2. Science, 2022. 378(6626): p. 1305-1315.
17. Kinisu, M., et al., Klf5 establishes bi-potential cell fate by dual regulation of ICM and TE specification genes. Cell Rep, 2021. 37(6): p. 109982.
18. Lin, S.C., et al., Klf5 regulates lineage formation in the pre-implantation mouse embryo. Development, 2010. 137(23): p. 3953-63.
19. Li, L., et al., Lineage regulators TFAP2C and NR5A2 function as bipotency activators in totipotent embryos. Nat Struct Mol Biol, 2024.
20. Iturbide, A., et al., Retinoic acid signaling is critical during the totipotency window in early mammalian development. Nat Struct Mol Biol, 2021. 28(6): p. 521-532.
21. Knudsen, T.E., et al., A bipartite function of ESRRB can integrate signaling over time to balance self-renewal and differentiation. Cell Syst, 2023. 14(9): p. 788-805 e8.
22. Uranishi, K., et al., Esrrb directly binds to Gata6 promoter and regulates its expression with Dax1 and Ncoa3. Biochem Biophys Res Commun, 2016. 478(4): p. 1720-5.
23. Olivieri, D., et al., Cooperation between HDAC3 and DAX1 mediates lineage restriction of embryonic stem cells. EMBO J, 2021. 40(12): p. e106818.
24. Herchcovici Levy, S., et al., Esrrb is a cell-cycle-dependent associated factor balancing pluripotency and XEN differentiation. Stem Cell Reports, 2022. 17(6): p. 1334-1350.
25. Carbognin, E., et al., Esrrb guides naive pluripotent cells through the formative transcriptional programme. Nat Cell Biol, 2023. 25(5): p. 643-657.
26. Zhang, M., et al., Esrrb Complementation Rescues Development of Nanog-Null Germ Cells. Cell Rep, 2018. 22(2): p. 332-339.
27. Hackett, J.A., et al., Tracing the transitions from pluripotency to germ cell fate with CRISPR screening. Nat Commun, 2018. 9(1): p. 4292.
28. Home, P., et al., Genetic redundancy of GATA factors in the extraembryonic trophoblast lineage ensures the progression of preimplantation and postimplantation mammalian development. Development, 2017. 144(5): p. 876-888.
29. Yamane, M., et al., Overlapping functions of Kruppel-like factor family members: targeting multiple transcription factors to maintain the naive pluripotency of mouse embryonic stem cells. Development, 2018. 145(10).
30. Wu, Y., et al., Yeast cell fate control by temporal redundancy modulation of transcription factor paralogs. Nat Commun, 2021. 12(1): p. 3145.
31. Kastner, P., M. Mark, and P. Chambon, Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell, 1995. 83(6): p. 859-69.


 (No Ratings Yet)
(No Ratings Yet)