Role of fat body lipid transport in Drosophila neurodevelopment
Posted by Padmapriya Ajith, on 27 October 2025
I never imagined that tiny fruit flies could reveal so much about the brain and its functions until I spent my summer in Alex Gould’s laboratory at the Francis Crick Institute, UK, under the supervision of Victor Girard. During these two months of internship, I used Drosophila melanogaster as a model system to investigate the role of circulating lipids in brain development.
As a second-year undergraduate student, my internship at the Crick was an eye-opening experience. It gave me the chance to engage with talented researchers, become a part of a welcoming scientific co mmunity and gain first-hand insight into how research unfolds in the real world. The Gould lab focuses on understanding how the developing central nervous system (CNS) adapts to environmental challenges such as hypoxia and nutrient deprivation. This research direction resonated with my own interests in neurobiology, making the experience both relevant and inspiring.
Lipids are essential for the structural framework of cells. They are, however, not just the building blocks of cell membranes but are also vital for energy storage and cell signalling. In the development of the nervous system, they are particularly important as neurons and glia both require extensive membrane synthesis and remodelling. In all animals, lipids are secreted into the circulation in the form of particles containing lipids and proteins, which are called lipoproteins. In Drosophila, lipoproteins are secreted mainly by the fat body, an organ functionally analogous to the adipose tissue and liver in mammals. My project aimed to investigate the role of fat body-derived lipoproteins in the neurodevelopment of Drosophila. To do this, I disrupted lipoprotein metabolism at two different scales: first by preventing the secretion of lipoproteins from the fat body and second by disrupting the local uptake of lipoproteins by the CNS. The goal was to measure the impact of these lipoprotein alterations on systemic and CNS growth, by measuring larval weight and larval brain volume respectively. To disrupt lipoprotein secretion from the fat body, I targeted two different genes via RNA mediated interference (RNAi): lipophorin (apolpp) and microsomal triglyceride transfer protein (Mtp) using a specific fat body GAL4 driver (Lpp-GAL4). Apolpp is functionally similar to apolipoprotein B in mammals, maintaining the structural integrity of the lipoprotein and mediating cargo recognition at destination tissues. Mtp is located in the endoplasmic reticulum and is involved in loading lipoprotein particles with apolipoprotein. I used a Drosophila transgenic line carrying a copy of apolpp tagged with green fluorescence protein (GFP) under its native promoter (apolpp::GFP), which acts as a fluorescent reporter of lipoproteins. I observed that RNAi knockdown of Mtp or Lpp in the fat body strongly decreased larval weight, thus indicating that fat body lipoproteins are critical for systemic growth (Fig.1A-B). In addition, Lpp or Mtp knockdowns severely reduced apolpp::GFP fluorescence in the hemolymph, the functional equivalent of mammalian blood, confirming that apolpp lipoprotein secretion from the fat body into the hemolymph is decreased in both conditions (Fig. 1C). Notably, upon Mtp knockdown, apolpp::GFP signal appears to be retained in the fat body.
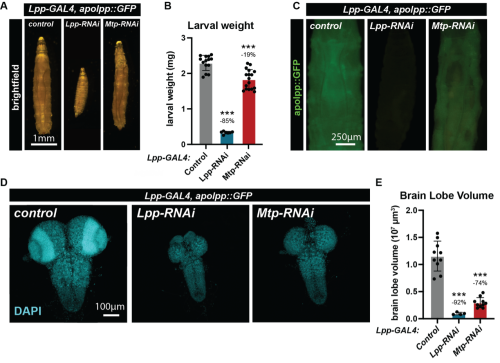
| Figure 1: Fat Body-derived lipoproteins are critical for systemic and brain growthA) Brightfield images of larvae of the control genotype or knockdown of Lpp-RNAi or Mtp-RNAi under the fat body driver (Lpp-GAL4). B) Weight of larvae 96 hours after larval hatching (ALH). C) Representative images of the distribution of lipoprotein reporter apolpp::GFP (green) in the indicated genotypes. D) Confocal micrograph of larval CNS nuclei stained with DAPI (cyan) of the indicated genotypes. E) Quantification of brain lobe volume. |
I then focused on the CNS, by measuring brain lobe volume as a proxy for its growth. I observed that fat body-specific RNAi knockdown of Lpp or Mtp dramatically reduced brain lobe volume (Fig. 1 D-E). Mtp knockdown had a stronger impact on brain volume than larval weight, suggesting that the developing CNS is particularly sensitive to low levels of circulating lipoproteins. Previous work from Suzanne Eaton’s lab has shown that lipoproteins are able to cross the blood-brain barrier (BBB). In Drosophila, the BBB is formed by a specialized subtype of glia that insulates the brain and regulates metabolite exchange with the surrounding hemolymph. I hypothesized that delivery of lipoproteins to the brain may require the lysosome, an organelle responsible for degrading endocytosed cargos. Specifically, I impaired lysosomal acidification by knocking down several subunits of the vacuolar H+ ATPase (V-ATPase) complex in glia and assessed the consequence upon systemic and brain growth. The knockdown of 5 different V-ATPase subunits had a limited impact on the overall weight of the larvae (Fig. 2A). Strikingly, knockdown of four out of the five subunits (Vha16-1, Vha26, Vha68-2 and Vha44) significantly reduced brain lobe volume compared to the control group (Fig. 2B). The fact that brain volume is strongly reduced but not the overall weight of the larvae suggests that lysosomal degradation is important for lipoprotein processing in the brain. To test this hypothesis, I then investigated the apolpp-GFP fluorescent reporter of lipoprotein at the blood brain barrier using confocal microscopy.
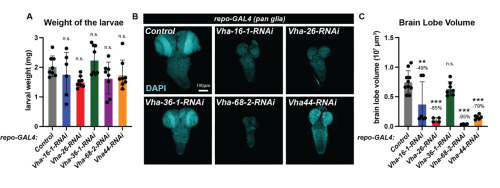
| Figure 2: Disruption of lysosomal degradation in glia impairs brain growth. A) Weight of larvae 96 hours ALH for knockdown of indicated V-ATPase subunits in glial cells(repo-GAL4); B) Confocal micrograph of larval CNS nuclei stained with DAPI (cyan) of the indicated genotypes. C) Quantification of brain lobe volume. |
Notably, knockdown of the V-ATPase subunit in glia resulted in the accumulation of apolpp::GFP puncta in round vacuoles (Fig. 3, white arrowheads) suggesting that undegraded lipoprotein may accumulate in lysosomes. In the future, this could be tested by co-staining using a lysosomal marker.
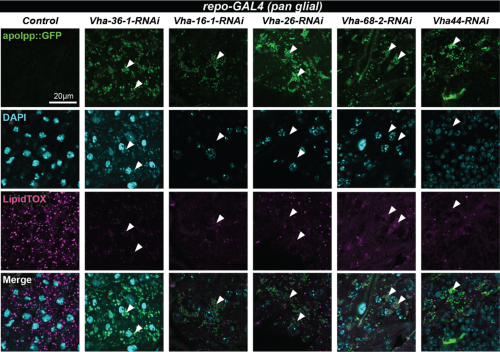
| Figure 3: apolpp accumulates in vacuoles in BBB glia deficient for V-ATPase subunitsConfocal images of blood brain barrier (BBB) glia of glial-specific knockdown of the indicated V-ATPase subunits. Lipoproteins are visualised with apolpp::GFP, a protein fusion under endogenous apolpp promoter (green), nuclei are labelled with DAPI (cyan) and lipid droplets with Lipidtox (magenta). Arrowheads point to cytoplasmic accumulation of apolpp::GFP in glial cells deficient for indicated V-ATPase subunits. |
This project provided me with a fascinating immersion into the process of scientific discovery and gave me a deeper appreciation for how model organisms can illuminate big questions in neuroscience. It has helped me cement my decision to pursue a research career in neuroscience. I am especially grateful to my supervisor, Victor Girard, for guiding me through this fascinating project and Alex Gould and the whole lab for their support and encouragement throughout the nine weeks. I would also like to extend my gratitude to The Francis Crick Institute and to the MRF Rosa Beddington Fund for funding my project and allowing me the opportunity to contribute to developmental biology research.


 (6 votes)
(6 votes)
Fascinating!
May I check if this is published anywhere (as in, are these results on any published journals)?
Hi, no its not published anywhere else.