Squishing jellies!!
Posted by Yamini Ravichandran, on 4 June 2025
Behind the paper: “Topology changes of Hydra define actin orientation defects as organizers of morphogenesis”
Long range order and topological defects as tissue shapers
My PhD focused on studying the role of CDC42 isoforms during cell polarization and migration1. As CDC42 is a well-known regulator of actin polymerization, I developed extensive training in understanding actin regulation and dynamics in the context of cell polarization and migration.
Towards the final year of my PhD, during my time at the Institut Curie in Paris, I encountered the captivating field of biological active matter. There, I was introduced to the works of several renowned biophysicists working at the intersection of physics and biology. I became deeply intrigued by the emerging studies in this field, which is largely driven by biophysicists exploring long-range order in biological systems and investigating whether singularities in this order—known as topological defects—act as organizational centers that facilitate key biological processes.
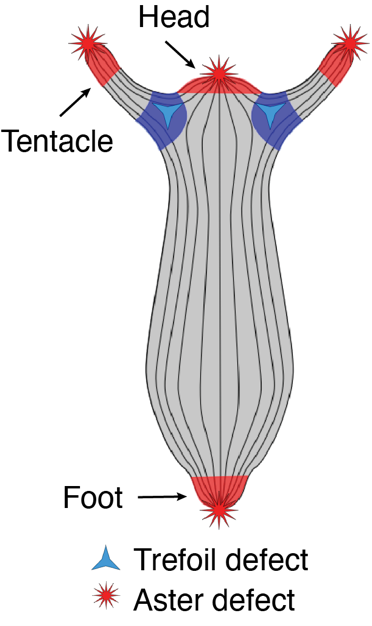
One striking example came from Benoit Ladoux, who demonstrated that cell extrusion events can occur at sites of topological defects in epithelial monolayers2. This was followed by two compelling preprints. The first, from the Roux Lab at UNIGE, showed that myoblasts confined to circular patches organize into nematic order (i.e., long-range, thread-like alignment). At aster-like topological defects, they observed cellular tornado-like 3D bulk cell extrusions, mimicking an in vitro reconstitution of muscle morphogenesis-like events3. The second preprint, from the Keren Lab in 2020, revealed that Hydra exhibits long-range nematic ordering in its supracellular actin organization, with topological defects correlating with head and foot morphogenesis4 (Fig. 1). Circling back to my PhD and my training in actin biology, seeing such single-cell-like highly organized actin structures in a tissue-scale regeneration in Hydra was fascinating and puzzling at the same time. I had so many agitating questions.
Beginning the postdoc: are topological defects shapers of in vivo morphogenesis?
I started in Aurélien Roux’s Roux Lab in September 2021—it was a rocky start, as I was still wrapping up my PhD manuscript and applying for postdoctoral fellowships, with deadlines fast approaching. I was also recovering from medical conditions that had worsened due to the sedentary lifestyle imposed on us during COVID.
In spite of these bottlenecks, I was genuinely excited to take on a new project to study the role of active matter in morphogenesis, especially in light of the recent breakthroughs in the field. Aurélien and I decided to explore the hypothesis of whether—and how—topological defects are required for shaping biological tissues, using various model organisms. We ventured into root morphogenesis in Arabidopsis (in collaboration with Luis Lopez Molina), slug morphogenesis in Dictyostelium (in collaboration with Thierry Soldati), and specifically, head regeneration in Hydra multi-headed mutants (in collaboration with Brigitte Galliot).
While I was juggling these different organisms and studying their fascinating morphogenetic events, I encountered a practical issue: my Hydra were constantly moving during imaging. I was performing these experiments with the help of Matthias Vogg, then a senior postdoc in the Galliot lab. To address the movement issue, I decided to image them using an agarose slab confinement method typically used for imaging plants—and Matthias agreed. Little did I know that this would compress the animal, leading to a mechanical induction of two-headed morphogenesis in Hydra.
Riding on this serendipitous discovery, I began following the phenomenon of mechanically induced morphogenesis in Hydra. During regeneration under compression, I tracked the emergence of new topological defects in the actin organization of the animal, which correlated with the formation of new heads. This observation confirmed the hypothesis proposed by the Keren lab, which suggested that aster topological defects are associated with new head formation during Hydra regeneration.
Topological defects shape animal tissues in a curvature dependent manner
Following this, Aurélien was thrilled and suggested I reach out to a theoretical physicist to explore the physical mechanisms behind how these additional topological defects could influence tissue shaping. He connected me with Daniel Pearce, an active matter theoretical physicist (then a postdoc in Karsten Kruse’s lab), who had already developed a mathematical model describing how long-range organization in Hydra could shape tissue5.
When I showed Daniel my findings, he was extremely excited and immediately came on board to help develop theoretical simulations of tissues under compression. Using his elastic nematic model, he described how +1 aster topological defects organize stresses and generate positive curvature (dome shapes), which is reminiscent of the shape of the Hydra head. In simulations under lateral compression, we observed that placing topological defects at the extremities, as seen in our regeneration experiments, led to the evolution of two dome-shaped structures.
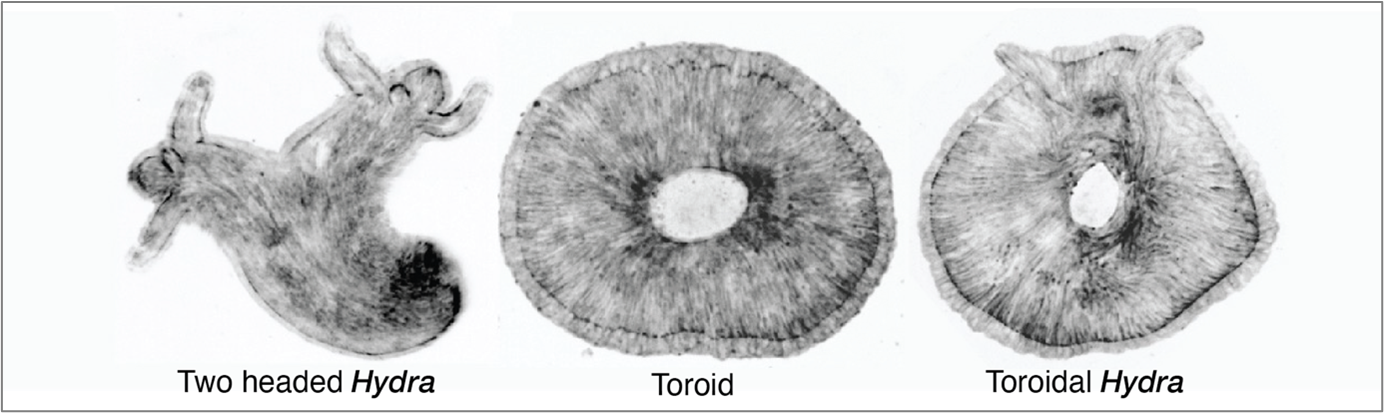
Furthermore, it was Dan who predicted that the orientation of tissue compression could dictate the fate of regeneration. The direction of compression influences how the long-range actin orientation experiences and responds to stress. As he suggested, we observed that when the tissue was oriented parallel to the compressive agarose slab, the sites of +1 aster defects buckled (inverted dome shapes) and underwent tearing, which then healed to form a defect-less toroid (Fig. 2).
This was a ground-breaking observation—it was the first time we observed the abolishment of body axis in an animal tissue that remained viable. I followed the defect-less torus over several days and observed that it failed to regenerate, as it maintained a perfectly symmetric actin configuration in which de novo +1 asters never arose. The tissue continuously attempted to regenerate a head but failed, due to the absence of a +1-aster topological defect.
Lastly, we all collectively thought however we should still be able to generate toroid with a +1 aster. By then we were also in touch with the Kerren lab and she invited me to Israel for a stay in her lab. Everyone suggested that theoretically a torus with defects could be generated. Then I hypothesized that if the compression occurred in a tissue with disordered actin to start with and a simultaneous tear occurred, then while the wound heals to create a 3D hole for the torus the disordered actin will order around it while generating a +1 aster required for a head. That is precisely what happened when we compressed the spheroid tissue that undergoes initial actin disorder and then ordering. This way we generated a torus with a +1 aster therefore a toroidal adult Hydra animal. This was really the cherry on top to see an animal with such a twisted muscle organization and topology.
In conclusion, these experiments established the need for +1 aster actin topological defects as head shapers in the animal. Their presence shapes the head and their absence give rise to no head formation, as observed in the defectless torus. The prospective research is to try to identify such long-range physical morphogens in other organisms, and strengthen the understanding of biological active matter.


 (2 votes)
(2 votes)