Understanding signalling pathways involved in the development of definitive endoderm.
Posted by Karolina Narbutt, on 4 November 2024
During my time as a summer student at the Francis Crick Institute, I had the privilege of working in the Developmental Signalling Laboratory of Dr Caroline Hill. Under the mentorship of Dr Berta Font Cunill, I have gained an insight into the realities of cutting-edge scientific research and was able to contribute to experiments advancing the understanding of developmental biology.
Throughout embryonic development, as cells divide, they begin to specialise to later form diverse functional tissues. This is possible because, even though progenitor cells contain copies of the same genetic code, they express different sets of genes. These gene expression patterns are governed by various complex signalling pathways, which ultimately determine cell fate. The Hill Lab is interested in understanding how cells use specific signals to communicate with each other and their environment to drive the development of an organism and tissue specialisation. During gastrulation, this cellular communication results in the embryonic stem cells transforming into three distinct germ layers: endoderm, mesoderm, and ectoderm. As their subsequent patterning generates all future body structures, this process sets the stage for the functioning of the entire organism (Richardson et al., 2023).
In my research project, I was specifically interested in the mechanism of cell differentiation into definitive endoderm. This germ layer gives rise to the lungs, bladder, the majority of the digestive tract, as well as vital endocrine organs like the pancreas and thyroid (Fang & Li, 2022). Understanding the signalling pathways involved in endoderm differentiation is necessary to generate novel therapeutic solutions to diseases associated with endoderm-derived tissues, such as diabetes. This is possible by using human induced pluripotent stem cells (iPSCs) to generate endodermal cells, and possibly their derivatives, in vitro (Fang & Li, 2022).
To study the differentiation to endoderm, I cultured iPSCs for four days in differentiation media with addition of CHIR-99021 (5 µM) for 24h, and Activin A (20 ng/mL) for 72h, following a standard protocol (Fig. 1a). Based on previous publications (Diekmann and Naujok, 2015), I expected that throughout this process, cells would follow certain patterns of gene expression (Fig. 1b). After 72h of differentiation (Day 4), I fixed and stained the cells with antibodies recognising key markers of pluripotency (Oct4) and endoderm (Sox17). I imaged the cells (Fig. 1c), quantified the results and was able to establish that the protocol of interest results in about 80% of stem cells differentiating to endoderm cells (Fig. 1d), confirming what has been observed in published articles.
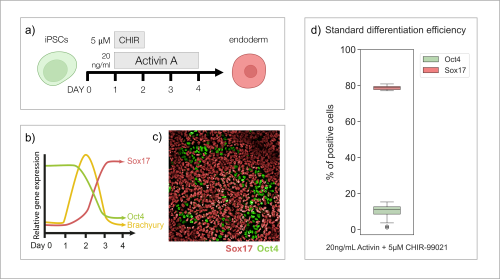
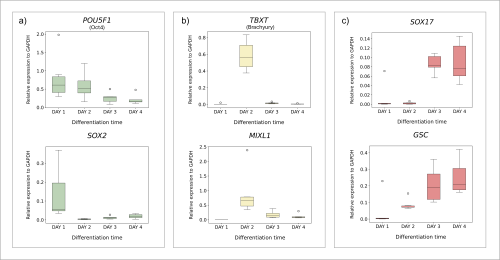
Having established the standard differentiation protocol, I wanted to understand the process itself in more detail. I set out to investigate the gene expression patterns throughout the transition of cells from pluripotency to endoderm. I collected cell samples on each day of the protocol. After extracting RNA, I synthesised cDNA to be used in quantitative polymerase chain reaction (qPCR) analysis. qPCR allows for the amplification of target DNA sequences, with simultaneous quantification of their concentration throughout the process. Thus, I was able to obtain and visualise the levels of expression of endoderm differentiation marker genes on each day of the protocol (Fig. 2). I was able to prove that cells transitioning from pluripotency to endoderm follow predicted patterns of gene expression (Fig. 1b). Pluripotency genes (POU5F1, SOX2) gradually decline as the differentiation continues (Fig. 2a), while endoderm markers (SOX17, GSC) are expressed more substantially towards the end of the process (Fig. 2c). I was also able to confirm that the cells go through an intermediate stage, with mesendoderm genes (TBXT, MIXL1) being expressed transiently on Day 2 (Fig. 2b).
To better understand the signalling pathways involved in the process of cell differentiation to endoderm, Dr Berta Font Cunill screened a library of over 1,000 small molecules of diverse molecular structure that could possibly affect the process by interacting with proteins important for endoderm differentiation. She identified one small molecule (compound “953”) that increases the differentiation efficiency to endoderm (from 80 to 90% approximately).
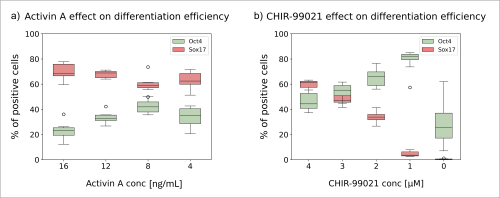
As I previously mentioned, after following the standard protocol the rate of differentiation to endoderm cells reaches about 80% (Fig. 1d). This high efficiency leaves a small margin for improvement. My goal was to modify the standard differentiation protocol to achieve a lower differentiation efficiency, and therefore increase the margin for improvement upon addition of compound “953”. I seeded pluripotent cells in media with varying concentrations of CHIR-99021 and Activin A (Fig. 3) and carried out the differentiation protocol for three days. I observed that even when the amount of Activin A was lowered from 20 ng/mL to 4 ng/mL, the differentiation proceeded only with minor changes in efficiency (Fig. 3a). However, lowering the amount of CHIR-99021 in just 1 µM increments hindered the process considerably (Fig. 3b). When CHIR-99021 was not present at all, most cells didn’t survive. These results show that CHIR-99021 is vital for endoderm differentiation.
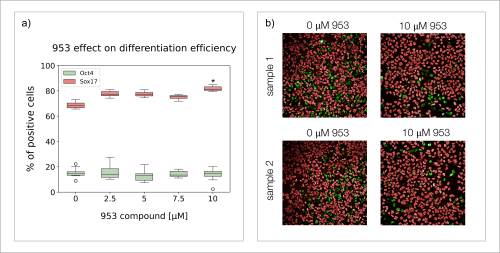
Based on the obtained results, I decided that the best condition to test the effect of compound “953” was 20 ng/mL of Activin A (48h), and 3 µM of CHIR-99021 (24h), which resulted in around 50% of differentiation efficiency, leaving a large margin for improvement. I cultured the cells for three days using these new conditions and different concentrations of compound “953”, after which I stained for Oct4 and Sox17 (Fig. 4). I observed that cells grown with 10 µM of compound “953” in the media, reached 10-15% higher differentiation efficiency than those grown without the compound (Fig. 4a). However, the final total number of cells was lower than in control groups (Fig. 4b). These results suggest that while molecule “953” pushes differentiation from pluripotency into endoderm cells, it is also possibly mildly toxic or hinders cell proliferation. The results also suggest that even if differentiation is hindered, compound “953” is not able to increase the differentiation efficiency beyond 10-15%.
The future of this project will focus on identifying the protein impacted by compound “953” through extensive proteomic analysis. This will allow for a better understanding of the signalling pathways involved in cell differentiation to definitive endoderm, which is a necessary step for the successful differentiation of downstream endoderm-derived tissues and organs to develop novel solutions in regenerative medicine. I am incredibly grateful for the opportunity to contribute to such inspiring scientific advancements. It has been an honour to be supported by the Rosa Beddington Fund. This experience has been a defining moment for my academic and professional development, and I have made the decision to pursue similar research through a PhD studentship and the rest of my scientific career. I would like to thank the Hill Lab, where I had the pleasure of working with incredible scientists, for their support and for welcoming me as a valued team member. I am especially grateful for the guidance and expertise of my supervisor, Dr Berta Font Cunill.
SOURCES
Diekmann, U., Naujok, O. (2015). Generation and Purification of Definitive Endoderm Cells Generated from Pluripotent Stem Cells. Methods in Molecular Biology, 1341, 157-72. https://doi.org/10.1007/7651_2015_220
Fang, Y., Li, X. (2022). Metabolic and epigenetic regulation of endoderm differentiation. Trends in Cell Biology, 32(2), 151-164. https://doi.org/10.1016/j.tcb.2021.09.002
Richardson, L., Wilcockson, S.G., Guglielmi, L. et al. (2023). Context-dependent TGFβ family signalling in cell fate regulation. Nature Reviews Molecular Cell Biology, 24, 876–894. https://doi.org/10.1038/s41580-023-00638-3
Silva, I.B.B., Kimura, C.H., Colantoni, V.P. et al. (2022). Stem cells differentiation into insulin-producing cells: recent advances and current challenges. Stem Cell Research & Therapy, 13, 309. https://doi.org/10.1186/s13287-022-02977-y
Zhao, M., Tang, Y., Zhou, Y. et al. (2019). Deciphering Role of Wnt Signalling in Cardiac Mesoderm and Cardiomyocyte Differentiation from Human iPSCs: Four-dimensional control of Wnt pathway for hiPSC-CMs differentiation. Nature Scientific Reports, 9, 19389. https://doi.org/10.1038/s41598-019-55620-x


 (12 votes)
(12 votes)