Unfinished story: How events during development affect adult behaviour
Posted by Smrithi Murthy, on 6 June 2025
Many times, the project or PhD is over, and the paper published, and yet the story unfinished. During the course of PhD, there arise many mysterious observations and unanswered questions.
My PhD work was published last year, where we describe the role of Cph (Chronophage) in locomotor behaviour of Drosophila. I report here an observation that isn’t completely explained by our present understanding of neural development in Drosophila.
Surprises on our path: When I started my PhD, with Prof. Upendra Nongthomba at the Indian Institute of Science, Bengaluru, this gene Cph was only a number (CG9650). Where to look for this unknown gene’s function? The project was a challenge, and it threw a couple of surprises in our path.
First, the only evidence that this gene might have a role in neural development (and hence our interest in it) came from a mis-expression screen. This screen showed that pan-neural expression of this gene i.e. in all neuroblasts and glia caused axon guidance defects. Was this due to its expression in neuroblasts/glia other than those in which it normally expressed? That’s what we thought – wrong cell, wrong time. The defects were grave enough to cause embryonic lethality.
However, upon knockdown of this gene in neuroblasts, or glia, or neurons, we didn’t see any axon guidance defect, or embryonic lethality. Knockdown in neurons resulted in flies that were constantly shaking, short-lived and failed to reproduce.
The second challenge lay in understanding the behavioural defect. I first thought the constant shaking of these flies was due to seizures. I requested inputs from experts – the late Prof. K. S. Krishnan (National Centre for Biological Sciences, Bengaluru, India), and Prof. Dan Kuebler (Franciscan University of Steubenville, Ohio, USA). Prof. Krishnan asked me to take a video of higher magnification. (Unfortunately he passed away before we could reach a conclusion). Prof. Dan Kuebler, an expert in epilepsy in Drosophila, said the shaking didn’t look like seizures to him.
It occurred to me that the flies that lie on their back and keep shaking are perhaps trying to get back on their feet. Most of them were unable to stand up, and even if they did, they would fall down within seconds. So, the shaking was due to a motor defect, and not seizures. Electro physiological recordings also supported this – aberrant spontaneous spikes indicative of seizure were absent. (However, they did show bang sensitivity i.e. when subjected to a mechanical shock, they went into paralysis. This is mostly seen in seizure susceptible genotypes. So seizure susceptiblility cannot be ruled out in Cph knockdown animals.)
The baffling observation: We asked – at what stage of development was this gene necessary? Surprisingly, knockdown of this gene in adults didn’t have any effect! Knockdown during the larval stage reduced locomotor activity at that stage, but abolished locomotor activity in adults.
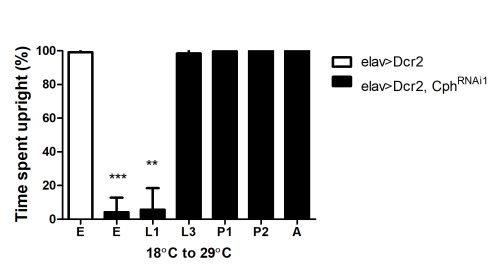
Effect of Cph knockdown at different stages of development: Cph was knocked down during different stages, and the resulting flies were assessed for their time spent upright.
Animals were initially reared at 18°C, and then shifted to 29°C at different stages of development. Shifting Cph knockdown animals at embryonic or L1 stage results in flies with locomotor defects. Shifting the animals post L3 stage results in flies with normal locomotor activity.
E, Embryo; L1, first larval instar; L3, third larval instar; P1, early pupa (less than 30hrs); P2, late pupa (70-90 hrs); A, adult
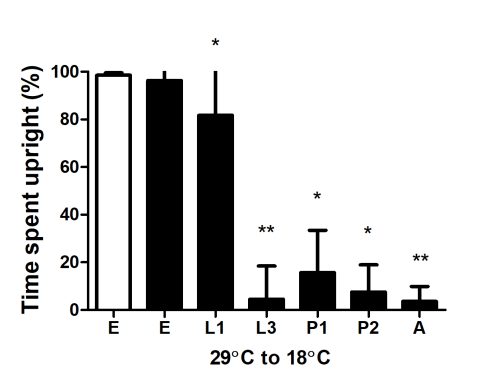
Animals were initially reared at 29°C and then shifted to 18°C at different stages of development. Shifting Cph knockdown animals at embryonic or L1 stage results in flies with normal locomotor activity. Shifting post L3 stage results in flies with locomotor defects.
How could this be explained? Were this protein expressed in larval neural stem cells, a possible explanation could be arrived at. But our observation was the result of knockdown in neurons! How could neuronal activity during development or the lack thereof affect their function in adults?
I outline two hypotheses:
- Effect on re-specification of primary neurons.
Primary neurons are those born during the first phase of neuroblast divisions in the embryo. These neurons function during the larval stage and are reconfigured to connect to new targets to function during the adult stage. During neuronal remodelling that occurs during metamorphosis, neuronal processes are withdrawn from the larval targets and new axons and dendrites form towards adult specific targets. This re-specification of primary neurons could be affected if their functioning during the larval stage caused irreversible changes in them.
In case of motor neurons, activity dependent plastic changes during the larval stage could affect neuromuscular junction morphology, and this effect could persist during the remodelling stage.
- Effect of energy state on formation/function of secondary neurons
Quiescent embryonic neuroblasts, post activation in first instar larvae, undergo another round of proliferation. This phase of neuroblast divisions produces the secondary neuronal lineages that function in the adult brain. Reactivation and subsequent division of these quiescent neuroblasts depends upon a signal from the fat body, which in turn depends upon the nutritional state of the animal (Chell and Brand 2010; Sousa-Nunes et al. 2011). Whether nutritional state also affects differentiation of neurons is not known.
Synapse function is an energy intensive process. ATP is required for axonal transport, vesicle fusion, neurotransmitter uptake etc. Animals with reduced PGK (PhosoGlycerateKynase), an enzyme required for ATP generation, show locomotor defects at larval and adult stage (Wang et al. 2004). PGK deficiency in humans has been shown to cause seizures, myopathy and muscle fatigue (Tsujino, et al. 1995), (DiMauro, Dalakas et al. 1983). The metamorphosis that follows the larval stage occurs using the energy stored during this stage. Metamorphosis is triggered by ecdysone release, which happens only after sufficient nutrients to survive metamorphosis have been acquired (Di Cara and King-Jones 2013). Though Cph knockdown animals probably acquire sufficient energy to survive metamorphosis, their neuronal development during this phase might be compromised.
I would welcome other ideas or suggestions.
References:
- Chell, J. M. and A. H. Brand (2010). “Nutrition-responsive glia control exit of neural stem cells from quiescence.” Cell 143(7): 1161-1173.
- Sousa-Nunes, R., L. L. Yee, et al. (2011). “Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila.” Nature 471(7339): 508-512.
- Wang, P., S. Saraswati, et al. (2004). “A Drosophila temperature-sensitive seizure mutant in phosphoglycerate kinase disrupts ATP generation and alters synaptic function.” J Neurosci 24(19): 4518-4529.
- Tsujino, S., S. Shanske, et al. (1995). “Molecular genetic heterogeneity of phosphoglycerate kinase (PGK) deficiency.” Muscle Nerve Suppl 3: S45-49.
- DiMauro, S., M. Dalakas, et al. (1983). “Phosphoglycerate kinase deficiency: another cause of recurrent myoglobinuria.” Ann Neurol 13(1): 11-19.
- Di Cara, F. and K. King-Jones (2013). “How clocks and hormones act in concert to control the timing of insect development.” Curr Top Dev Biol 105: 1-36.


 (No Ratings Yet)
(No Ratings Yet)