Unravelling plant surface appendages
Posted by Rishan Singh, on 19 August 2024
Abstract
Plant surface appendages may exist in different shapes, size and conformations. With each variation comes a different or overlapping function. However, plant surface appendages, or trichomes, are an advantage to any plant having them. Here, plant surface appendages will be unraveled with reference to plant species and the role they play within the ecological niche.
Keywords: frost, heat, fruit, gravity, calyces, photorespiration, forage, Venezuelan plants
There are a finite number of mystical structures, called plant epidermal appendages, on the surface of different plant species. These appendages are the first point of contact that plants have with carbon dioxide in the ecological niche (Johnson, 1975; Singh, 2017; Singh, 2018). But what does this mean? It simply implies that plant epidermal appendages enable optimal functioning of the plants in their environment (Johnson, 1975; Ehleringer, 1984). During seasonal changes, and overlaps, these appendages help plants sustain themselves, and this is irrespective of whether the plant is floral, ornamental, or even edible. Since plant epidermal appendages contain a fair amount of cytoplasm, windy conditions aren’t much of a threat because they can change their conformation from flubbery to stiff, and vice-versa (see Figure 1 as an example). Singh (2017) has reported that cytoplasm within trichomes (i.e. plant epidermal appendages) arose from protoplasmic evolution during plant growth. This has made their point of contact with the external environment a unique trait, particularly because of the epidermal basal cells (Ali and Al-Hemaid, 2011; Duffey, 1986; Tooker et al., 1986).
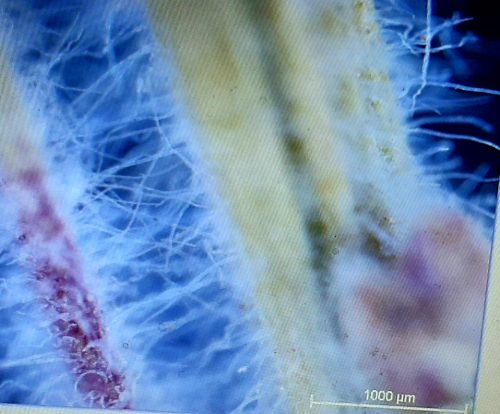
Figure 1: Stereomicrograph showing the hair-like protrusions in a bottlebrush stem.
There are different types of trichomes, and these differ in arrangement, shape and size (see figure 2). According to Singh (2024), the arrangement of trichomes determine the mechanism for homeostatic control of temperature and photosynthesis in plants. So what is the indirect control of photosynthesis? The indirect control of photosynthesis is achieved by the trichomes protecting photosystems, thylakoid membranes and even the grana. In Fragaria sp. (strawberry plants), trichomes are found to be scattered over leaf surfaces, but don’t play an important role in water conductivity of plants. However, despite this setback, the plant surface appendages help maintain the function of the xylem and phloem tissues through its ecological roles (Singh, 2017). In Cucurbit sp, on the other hand, the epidermal appendages offer a third level of protection to the plants by preventing large creatures from feasting on the crunchy, water-filled, stems (Singh, 2018; Singh, 2024). So what’s an interesting feature in butternut and pumpkin plant species? In Cucurbita moschata, there is a physical growth mechanism. In this plant, the gravitational pull offered by the trichomes enable them to remain on the ground (Singh, 2018). However, there are far more roles, arrangements and sizes than this. Venezuela mahogany (Swietenia macrophylla) have fine pubescent trichomes on young leaves, while those found in Palo Santo (Bursera graveolens) are short and glandular. In Palo Santo, the plant epidermal appendages occur on the leaves and stems, just like with Venezuelan Solanum species, e.g. Solanum griseum, however, in Solanum griseum, the trichomes are dense and stellate. With Capsicum annuum, i.e. chilli pepper, the trichomes are non-glandular and short. From this, all 4 Venezuelan plant trichomes protect the plants against physical and mechanical damage, which may be imposed by predators. They also help the plants minimize water loss (Singh, 2024).
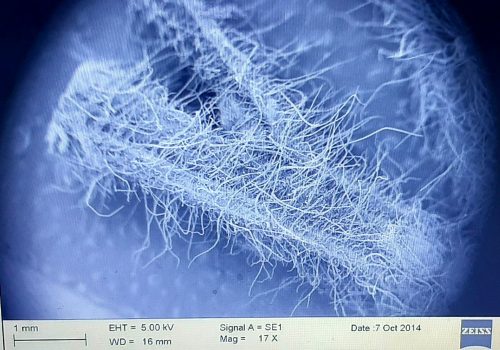
Figure 2: Scanning Electron Micrograph showing different length and arrangement of trichomes on a bottlebrush stem with a petiole.
When experimental researchers are lost, they tend to use trichomes as a means of finding their way back in the field. This role is prominent during field experiment studies (Singh, 2017; 2018). Tooker et al. (2010) & Kesslet and Baldwin (2002) has reported that trichomes offer warmth and protection to plants during cold and frosted conditions, and that they may also provide shade for plants during extreme weather conditions. Therefore, during night and day, the trichomes enable the maintenance of gradients of sodium and potassium across the stomatal aperture (Singh, 2018). In evolutionary terms, plant surface appendages are an advanced trait (see Figure 3), because they provide a barrier to danger which plants may encounter in the environment (Duffey, 1986; Tooket et al., 2010; Kessler and Baldwin, 2002; Wagner et al., 2004).
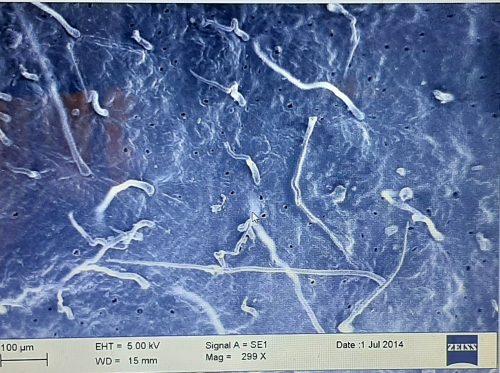
Figure 3: Scanning electron micrograph showing epidermal appendages protruding from the surface of a leaf.
Duffey (1986) says that plant epidermal appendages invoke fear in farm and field animals. They are known to prevent the ingestion of plants by herbivorous and omnivorous predators. In this way, natural selection through herbivory and omnivory is prevented (Singh, 2018; 2024), allowing longevity of plants in the wild. In bud calyces, trichomes have a common role in protecting flowers. In addition, it is through plant epidermal appendages favoring pollination and seed dispersal that angiosperm and gymnosperm plants have a competitive growth advantage. Singh (2024) reports that Actinidia deliciosa, also known as Kiwi plants, have appendages on their surface which protect the fruit from herbivores and fruit forages by camourflaging the fruit. Singh (2024) further mentions the points that follow— In Nettle, Urtica dioica, the trichomes are needle-like and have the capacity of injecting irritating compound. However, as much as the trichomes protect the plant from herbivores, they also provide a safe habitat as well as food for some insects. In contrast, in Lamb’s ear (Stachys byzantina), the surface appendages are dense, soft and velvety. In this plant, the trichomes reflect sunlight and reduce water loss by trapping moisture. Since they are prone to herbivore foraging, the trichomes serve as an ecological trait that protects the plant by deterring them. In wide contract to the above, Phaseolus vulgaris, also known as the common bean, possesses short glandular trichomes that are apt to protect the plant against herbivores (read Singh, 2017). It’s been reported that the trichomes not only produce substances to keep herbivores away, but that they also have antimicrobial properties that protect the plant from pathogens. Just like the common bean, tomato plants (Solanum lycopersicum) have glandular trichomes. However, the trichomes produce a resin that trap insects and protect the plant against herbivores. This is a kind of defense mechanism in Solanum lycopersicum. Moreover, the trichomes limit transpiration; and this prevents water loss. Similar to the function of trichomes in tomato plants, Cucumis sativus (Cucumber) trichomes possess the same function. However, the plant surface appendages are non-glandular and bristly. Since they are non-glandular, the trichomes are more afforded for the purpose of trapping dust and debris, thereby reducing pathogen density. In conclusion, the unveiling of the uniqueness of plant epidermal appendages is a tremendous endeavor and is ongoing throughout the world.
Acknowledgement
I would like to thank the Electron Microscopy Unit at the University of KwaZulu-Natal, Westville campus for being able to capture the micrographs showing hair-like structures.
References
Ehleringer J. (1984). Ecological and ecophysiology of leaf pubescence in North American dessert plants, in: Rodriquez E., Healey PL., Mehta I. (eds.), Biology and Chemistry of Plant Trichomes, New York: Plenum Press, pp. 113-132.
Ali MA., Al-Hemaid FMA. (2011). Taxonomic significance of trichomes micromorphology in Cucurbits. Saudi Journal of Biological Sciences 18 (1): 87-92.
Duffey SS. (1986). Plant glandular trichomes: their partial role in defence against insects, in: Juniper BE, Southwood TE. (eds.), Insects and the Plant Surface, London: Arnold, pp. 151-172.
Johnson H.B. (1975). Plant pubescence: an ecological perspective. Botanical Review 41, 233-253.
Kessler A., Baldwin IT. (2002). Plant responses to insect herbivory: the emerging molecular analysis. Annual Reviews in Biology 53: 299-328.
Singh R. (2017). A review, outlook, and insight, of the properties and characteristics of Callistemon citrinus. Bulletin of Pure and Applied Sciences – Botany 36b(1): 22-27.
Singh R. (2018). A commentary on the hair-like (indumentum) structures in the leaves of African pumpkin, Cucurbita maxima. Bulletin of Pure and Applied Sciences- Botany 35b: 35-41.
Singh R. (2024). pers writing, RSA.
Tooker JF., Peiffer M., Luthe DS., Felton GW. (2010). Trichomes as sensors: Detecting activity on the leaf surface. Plant Signal Behaviour 5 (1): 73-75.
Wagner GJ., Wang E., Sheperd RW. (2004). New approaches for studying and exploiting an old protuberance, the plant trichomes. Annals of Botany 93 (1): 3-11.


 (No Ratings Yet)
(No Ratings Yet)