Ancient bones in fossils and embryos of living dinosaurs
Posted by Daniel Smith-Paredes, on 20 December 2018
Birds are a dominant group of land Vertebrates (probably the largest in numbers with +10000 species described), highly successful and diverse. Birds originated from members of the Theropoda: the meat-eating dinosaurs that included famous forms like T. rex or Velociraptor, well-known from the movies. The fact that birds are a kind of dinosaur has been a matter of debate not except from controversy, but largely accepted today. The two most important lines of evidence that allowed us to understand the true identity of birds have been the (incredibly detailed) fossil record of the non-avian to avian dinosaur transition, and birds’ embryological development. Embryology reveals the dinosaur within birds and in cases development can parallel in short time what we see to have happened in millions of years of fossil record. Alexander Vargas’s lab at the University of Chile focuses on this evolutionary transition, employing both fossils and embryos to understand the workings of evolution.
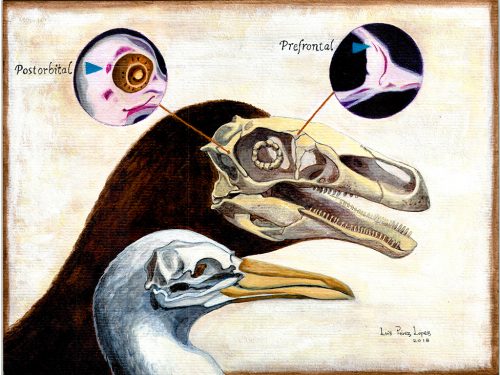
Researchers of comparative anatomy have documented how embryos show the dinosaur-bird link since the times of Darwin, mostly focusing on the postcranial skeleton. With this idea in mind, we set to study the development and evolution of the dinosaur skull. The head of birds is very unique, and two key differences when comparing birds to other reptiles are their skull and the huge brains and eyes birds possess. The bones composing the skull of birds are thin, light and mostly fused together in the adult. In addition, two bones (the postorbital and the prefrontal, located behind and in front of the eye respectively), were lost at different moments during the evolution of the dinosaurs leading to modern birds. Both in the fossil record and during embryonic time, how and when these bones disappeared, and if there were remnants of their presence as suggested by previous reports, were some of the questions we wanted to resolve in our recent paper in Nature Ecology and Evolution.
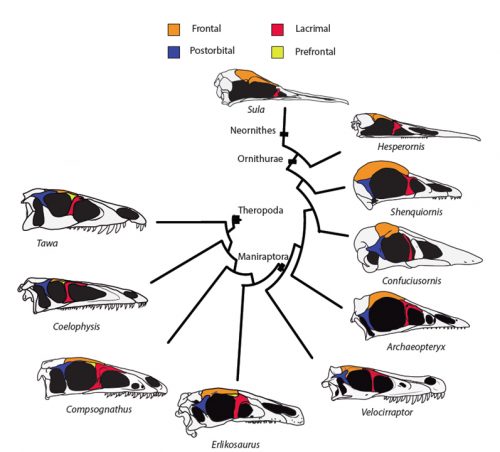
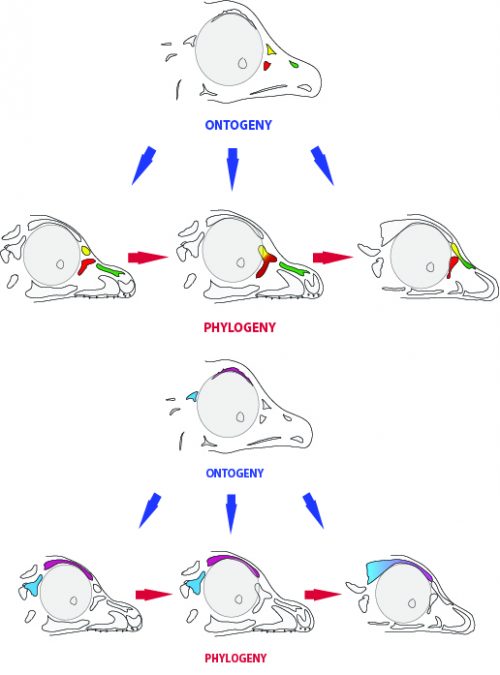
By looking at the fossil record of Theropods, we found two distinct “moments” when these bones were lost, at the origin of two recognizable clades: the postorbital was lost at the Euornithes node, close to modern birds; while the prefrontal was lost at the Pennaraptora node, right at the base of the clade containing dinosaurs like Oviraptor, Velociraptor, Archaeopteryx and modern birds. This bone has an interesting story, as in most early members of this clade, the prefrontal was not present, while the lacrimal bone acquired a T-shape (in contrast to the inverted L-shape of other dinosaurs that did possess a prefrontal). This T-shape, in which the T’s posterior tip occupies the position otherwise used by the prefrontal, suggested these bones were the result of fusion between the prefrontal and the lacrimal. The surprising part was that some dinosaurs seemed to be reverting into having a separate prefrontal bone. Some specimens of different species of these dinosaurs were showing a separate prefrontal bone, while at the same time losing that “tip” of the lacrimal that could have been the fused prefrontal. Even members of the same species would show variability in the presence or absence of the adult bone, as Deinonychus or Archaeopteryx specimens could have a prefrontal separated. To understand the basis of these changes in the cranium, we went to embryos.
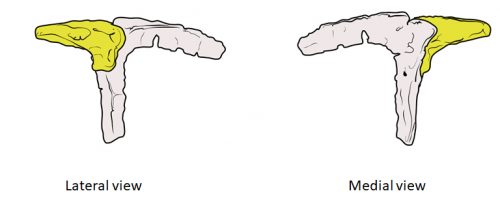
Chicken embryos have been a preferred animal model for those willing to study embryonic development for more than 2000 years, and have provided the vast majority of the information we have today about skull development for birds. However, they are just one species among thousands, representing one of hundreds of distinct lineages. In an effort to include more of the disparity and diversity of birds, we collected embryonic series of six families of birds, including (of course) chicken, but also ducks, and species not normally used for developmental studies like lapwings, coots, budgerigar and tinamous (a member of the palaeognathae related to ostriches and emus), and also included embryonic stages of Alligator mississippiensis to broaden our sample to members of the two living archosaur groups, birds and crocodylians. By looking at the avian embryos and their developing ossifications alone, we confirmed the presence of more ossification centers than adult bones in the embryos of birds, but only by comparing them with alligator embryos and the fossil record were we able to interpret them in light of their evolutionary history.

In embryos of all the birds observed, two ossification centers develop and make up the adult lacrimal bone. These two ossifications were observed and identified as the lacrimal and prefrontal bones of chicken by Erdmann in 1940, but we also found two separate ossifications giving rise to the lacrimal of alligator embryos, which do form a separate prefrontal from another ossification center, meaning Erdmann’s identification of a prefrontal bone in the chick was mistaken. However, we did find an embryonic bone in one species, the Chilean tinamou, where in addition to the two ossification centers of the lacrimal, a third, well-developed ossification develops in a position similar to that of the embryonic Alligator and adult dinosaur prefrontal. This bone, however fused to the nasal bone, and becomes indistinguishable from its other pieces. In a way, the bone is doing what we think it did in dinosaurs like Velociraptor: developing as a separate center and later fusing to a neighboring bone. This separate embryonic origin can explain why it re-appeared in different specimens as a separate adult bone, as it likely failed to fuse. However, in the tinamou, it is doing something it did not do in Velociraptor as instead of the lacrimal, it is fusing to a different neighbor.
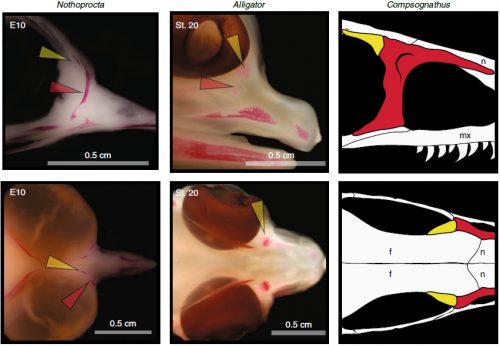
We also found an “extra” ossification not corresponding to any adult avian bone and just behind the eye that looks like the embryonic postorbital ossification of Alligator and the adult postorbital bone of dinosaurs, but instead of remaining separate as in these animals, fuses to the back of the frontal bone.
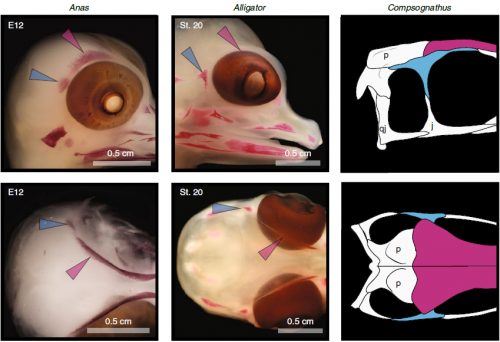
In a way, we were expecting to find this ossification, since the frontal of birds has been described as being formed from two separate portions, derived of two distinct embryonic germ layers; the mesoderm and the neural crest. Bones in the skull of vertebrates come in two flavors, as the most-rostral ones derive from neural crest cells and the ones in the back of the skull come from the mesoderm. We have known of this for years, thanks to careful chick-quail chimera experiments, and the double origin of the frontal has in fact created a lot of debate on the frontal identity itself. In other vertebrates, bones usually derive from one of these embryonic sources, and the frontal in particular is made up cells of neural crest origin. While other researchers also proposed the mesodermal portion could correspond to a separate bone that ended up fusing to the frontal, the identification of this portion as the parietal bone did not agree with many morphological criteria, anatomical correspondences nor with the evidence presented by the fossils. Our suggestion that the back portion of the avian frontal comes from the ossification that gives rise to the postorbital in other reptiles is consistent with the compared embryology of birds and crocodylians, as well as with the fossil record. This proposed homology implies the postorbital of Alligator should derive from mesoderm cells, something not yet corroborated as fate mapping of crocodilian embryos has not yet been done.
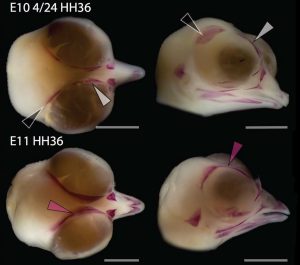
The history of transformations and fusions of the prefrontal and postorbital provides us with some interesting lessons on how evolution takes place, but also leaves us with a number of intriguing questions of how skull development is regulated. Upon fusing with a neighboring bone, both the prefrontal and postorbital apparently lost their own identities, becoming a non-independent part of the larger bone, not showing any morphological similarity to the separated bone of other species. Moreover, the cells that make up the independent ossification centers end up forming a structure in which there’s no trace or clue of their origin, even when coming from two very distinct embryonic sources as are the neural crest and mesoderm. Contrary to most of the bones in the body, which form a mold or cast of cartilage that is later replaced by bony tissue, many bones in the skull (particularly those of the face, skull roof, palate and jaws) develop directly into bone from mesenchymal condensations. We know a (staggering) lot more about how these cells invade the head and face or find their location than what we know about how the mesenchymal condensations are established and how they turn into individual bones. Between the migration of neural crest cells into the head, for example, and the onset of bone formation, there’s a gap in our understanding that has still to be bridged. Questions like what signals regulate the condensation mesenchyme, the beginning of bone formation or the spatial distribution of the ossification centers that form the bones are still poorly answered. Our knowledge on this kind of bone development derives from (and has been limited by) histological sections, which do not allow us to have a whole-picture of what’s going on in the whole head, and the study a few molecular markers that mostly label post-mesenchymatic condensation and pre-osteogenic stages of maturation. It is, in truth, by these limitations that our study relied only on bone staining to observe and compare the ossification patterns of different species. More understanding of the whole picture was not going to come from digging into deeper molecular mechanisms in the chicken, but from studying and comparing the development of more species, in a maybe simpler way, like Alizarin staining.
One interesting thing to consider is that one possible driver behind the huge modification of the skull in the evolution of birds might be the evolution of a big brain. Birds have enlarged brains compared to other reptiles and non-avian dinosaurs, and the enlargement of the brain would necessitate a re-structuring of the skull covering it. Other cases of evolution of big brains also result in evolution of the skull as a whole, and even loss of bones, maybe in a similar way than what we see in birds. In mammals, bones of mixed origin proved to be composites resulting from fusion of elements, including those supposedly lost long ago in the earliest mammalian lineage. Although these bones are located in the back of the skull, mammals also lost bones like the prefrontal or postorbital. The brain, being a huge structure lying directly underneath the developing skull-roof, can possibly influence mesenchymal condensations, bone deposition, ossification rate and overall timing and spatial development of the skull. How these structures interact and how they evolve in concert is yet to be studied and understood.
One last lesson we can derive from our results is the evolutionary meaning of the embryonic persistence of a structure. The ossification centers we found add to a longer list of stories in which the embryo retains at least a rudiment of a long-lost structure, which enables the evolution of new morphologies in later lineages. By retaining the prefrontal ossification, for example, some dinosaurs were (and still are) able to “experiment” a variety of morphological outcomes that include fusion with the lacrimal or nasal bones. It also worth noting that, at least in the case of the skull of birds, the adult bones end up fused together. So in a sense, the order or pattern in which ossification centers are beginning to fuse to each other could be less of adaptive significance than it is just a phenomenon of developmental drifting, in which a pattern is established and conserved. Itself, the high degree of bone fusion in the avian adult skull could be an extension of a trajectory started by those dinosaurs in which first two bones, then two others began fusing.
How does evolutionary change take place as shown by the fossil record and during embryonic time can be a question not just of “how dinosaurs looked like” but also an important source of understanding of developmental mechanisms at play every day.
- Erdmann, K. (1940). Zur Entwicklungsgeschichte der Knochen im Schädel des Huhnes bis zum Zeitpunkt des Ausschlüpfens aus dem Ei. Zeitschrift für Morphologie und Ökologie der Tiere, 36(3), 315-400.
- Le Lièvre, C. S. (1978). Participation of neural crest-derived cells in the genesis of the skull in birds. Development, 47(1), 17-37.
- Noden, D. M. (1983). The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Developmental biology, 96(1), 144-165.
- Evans, D. J., & Noden, D. M. (2006). Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Developmental dynamics: an official publication of the American Association of Anatomists, 235(5), 1310-1325.
- Piekarski, N., Gross, J. B., & Hanken, J. (2014). Evolutionary innovation and conservation in the embryonic derivation of the vertebrate skull. Nature communications, 5, 5661.
- Maddin, H. C., Piekarski, N., Sefton, E. M., & Hanken, J. (2016). Homology of the cranial vault in birds: new insights based on embryonic fate-mapping and character analysis. Royal Society open science, 3(8), 160356.
- Abzhanov, A., Rodda, S. J., McMahon, A. P., & Tabin, C. J. (2007). Regulation of skeletogenic differentiation in cranial dermal bone. Development, 134(17), 3133-3144.


 (1 votes)
(1 votes)
Thank you for sharing such an interesting article.