Insights into the pathogenic role of UVRAG in intestinal dysplasia
Posted by Disease Models and Mechanisms, on 4 May 2016
This post highlights the approach and findings of a new research article published in Disease Models & Mechanisms: ‘Stem cell-specific endocytic degradation defects lead to intestinal dysplasia in Drosophila’. This feature was written by Elan Strange as part of a graduate level seminar at The University of Alabama (taught by DMM Editorial Board member, Prof. Guy Caldwell) on current topics related to use of animal and cellular model systems in studies of human disease. The course is designed to expose students to recent research in a variety of diseases, and for this assignment, students were asked to read and provide a scholarly summary of an assigned research article ‘in press’ at DMM. Elan’s summary was selected by the editorial team for publication at the Node. The text has been edited and shortened by DMM in conjunction with the author.
A useful approach to investigating the mechanisms underlying complex diseases such as cancer involves exploring common genetic mutations. Understanding the phenotypic impact of such mutations can help to identify risk, estimate prognosis and guide treatment for specific forms of cancer. For example, screening for BRCA1 and BRCA2 mutations has been shown to be effective in determining risk of developing breast and ovarian cancer (Mavaddat et al., 2013). Similarly, Marisa et al. (2013) showed that grouping colon cancer patients into subtypes based on genetic mutations can provide a better indication of prognosis. Researching genetic mutations that correlate with oncogenesis has proven to be an invaluable means of learning more about the causes of cancer and guiding the development of new chemotherapeutics.
UVRAG, the metazoan homolog of yeast Vps38, is well characterized as a regulator of autophagy (Liang et al., 2006), a conserved mechanism by which cells digest and recycle dispensable or dysfunctional organelles and cellular components. Although loss-of-function mutations in UVRAG are known to correlate with tumorigenesis (Ionov et al., 2004) and overexpression of the protein has been shown to reduce cell proliferation (Liang et al., 2006), the precise mechanisms by which UVRAG acts as a tumor suppressor have not yet been elucidated. Given that autophagy has been shown to be involved in several types of cancer (Bento et al., 2016), the most intuitive hypothesis for the role of UVRAG in tumorigenesis implicates its autophagy-regulating function. However, this hypothesis was explored by Knævelsrud et al. (2010), who determined using qualitative and quantitative readouts for autophagy that the tumorigenicity of UVRAG mutations in colorectal cancer cell lines is independent of its role in regulating autophagy. Additionally, UVRAG has functions in DNA repair, maintenance of centrosome stability, and endocytosis (Zhao et al., 2012), all of which are implicated in cancer and could explain the role of UVRAG as a tumor suppressor. In a new study published in DMM, Nagy et al. sought to investigate the role of UVRAG as a tumor suppressor, using the fruit fly Drosophila melanogaster. Drosophila represents a powerful model for exploring the pathology and molecular mechanisms of human intestinal disorders due to the highly similar histological and cellular stress response mechanisms (specifically those involving cell proliferation and renewal) in the guts of mammals and flies.
The authors began by using RNAi silencing to study the effects of adult-onset loss of Uvrag in Drosophila intestinal stem cells (ISCs). They report that the Notch ligand Delta and Wnt ligand Wingless (Wg), two biomolecules known to be trafficked via endosomes, accumulate in intracellular compartments of ISCs lacking UVRAG, thus indicating that these cells are deficient in endosomal trafficking. They validated their RNAi silencing experiments by showing that cells expressing null alleles of Uvrag show similar patterns of Delta and Wg accumulation.
The team then analyzed the effects of Uvrag silencing on ISC proliferation and morphology. The most noteworthy observations made were an increase in ISC number and concomitant thickening of the intestinal wall, both of which are characteristics of intestinal dysplasia (a precancerous lesion, see related DMM Review). To analyze the effects of silencing Uvrag on gut function, the authors fed the flies food containing a blue dye that enabled movement through the gut to be tracked. The feces of flies with UVRAG-deficient ISCs contained less dye, indicating
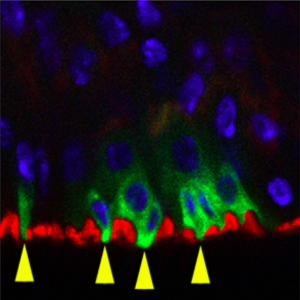
that these animals retain food more efficiently. Consistent with a previous finding that proper gut function is essential for normal lifespan (Biteau et al., 2010), Nagy et al. found that the fly mutants had significantly reduced lifespan. To look at how flies with UVRAG-deficient guts respond to environmental stress, the authors treated the flies with the toxin dextran sodium sulfate (DSS), and, in a separate experiment, infected them with the pathogen Pseudomonas aeruginosa. Under both treatments, UVRAG-deficient flies were killed faster than control flies. Overall, these experiments show that UVRAG deficiency induces gut dysplasia and sensitizes the gut to external stressors.
ISCs maintain integrity of the gut by proliferating and differentiating via a process dependent on Notch, which induces differentiation by activating the well-studied kinase, target of rapamycin (TOR) (Kapuria et al., 2012). This process involves individual ISCs undergoing asymmetric cell division to produce a new ISC and an enteroblast, the latter of which can then differentiate into an enterocyte (90% of the time) or an enteroendocrine cell (Zeng et al., 2011). The authors wanted to see if Notch signaling can regulate this process in the absence of UVRAG.They report that despite the presence of Notch activity in UVRAG-deficient cells, there is a significant lack of differentiation and active TOR. Interestingly, Uvrag silencing resulted in larger and selective impairment of enteroblast differentiation into enterocytes (but not into enteroendocrine cells).
Based on a previous finding showing that JAK/STAT regulates ISC proliferation in Drosophila (Jiang et al., 2009), the authors sought to determine the activity of key proliferation/differentiation signaling pathways in UVRAG-deficient intestines. They found that while AKT and Ras-MAPK pathways were not involved, JNK activity was misregulated in UVRAG-deficient ISCs. Subsequent knockdown of the JNK homolog Basket or STAT homolog Stat92E suppressed the hyperproliferation seen in UVRAG-deficient ISCs.
The authors then looked at how Notch signaling, which has been implicated in regulating ISC differentiation (Micchelli and Perrimon, 2006), is affected by Uvrag silencing. Silencing of both Uvrag and Notch suppressed ISC differentiation, while Notch overexpression rescued the impaired differentiation phenotype induced by Uvrag silencing. To address the question of whether or not the effects of Uvrag silencing are a consequence of autophagic defects in ISCs, the authors measured levels of the trafficked nucleoporin p62 homolog Ref(2)P. No differences in the endogenous levels of Ref(2)P in UVRAG-deficient and wild-type ISCs were detected, suggesting that autophagy is not impaired in ISCs in the absence of UVRAG.
In perhaps the most revealing experiment presented in the paper, the authors expressed a dominant-negative form of the dynamin homolog (shibire) and silenced Rab7 using RNAi (both in ISCs) to inhibit early and late endocytosis, respectively. Expression of dominant-negative shibire was lethal in young flies; however, silencing of Rab7 induced gut dysplasia in a manner that mimicked Uvrag silencing. This exciting result provides compelling evidence that intestinal dysplasia induced by knocking out Uvrag expression is a result of impaired endocytic trafficking.
The goal of this study was to learn more about the pathogenic role of loss-of-function mutation of UVRAG often observed in human colorectal cancer. The authors determined that deregulation of endocytic trafficking in ISCs, driven by loss of UVRAG, leads to intestinal dysplasia in Drosophila. Given that intestinal dysplasia is a common precancerous lesion in the human gastrointestinal tract, this finding provides important insight into the potential role of UVRAG in colorectal cancer.
References
Bento, C. F., Renna, M., Ghislat, G., Puri, C., Ashkenazi, A., Vicinanza, M., Menzies, F. M. and Rubinsztein, D. C. (2016). Mammalian Autophagy: How Does It Work? Annu. Rev. Biochem. 85, annurev–biochem–060815–014556.
Biteau, B., Karpac, J., Supoyo, S., DeGennaro, M., Lehmann, R. and Jasper, H. (2010). Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 6, 1–15.
Ionov, Y., Nowak, N., Perucho, M., Markowitz, S. and Cowell, J. K. (2004). Manipulation of nonsense mediated decay identifies gene mutations in colon cancer Cells with microsatellite instability. Oncogene 23, 639–645.
Jiang, H., Patel, P. H., Kohlmaier, A., Grenley, M. O., McEwen, D. G. and Edgar, B. A. (2009). Cytokine/Jak/Stat Signaling Mediates Regeneration and Homeostasis in the Drosophila Midgut. Cell 137, 1343–1355.
Kapuria, S., Karpac, J., Biteau, B., Hwangbo, D. and Jasper, H. (2012). Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Drosophila Intestinal Stem Cell Lineage. PLoS Genet. 8, 1–14.
Knævelsrud, H., Ahlquist, T., Merok, M. A., Nesbakken, A., Stenmark, H., Lothe, R. A. and Simonsen, A. (2010). UVRAG mutations associated with microsatellite unstable colon cancer do not affect autophagy. Autophagy 6, 863–870.
Li, H. and Jasper, H. (2016). Gastrointestinal stem cells in health and disease: from flies to humans. Dis. Model. Mech.
Liang, C., Feng, P., Ku, B., Dotan, I., Canaani, D., Oh, B.-H. and Jung, J. U. (2006). Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 8, 688–99.
Marisa, L., de Reyniès, A., Duval, A., Selves, J., Gaub, M. P., Vescovo, L., Etienne-Grimaldi, M. C., Schiappa, R., Guenot, D., Ayadi, M., et al. (2013). Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value. PLoS Med. 10, 1-13.
Mavaddat, N., Peock, S., Frost, D., Ellis, S., Platte, R., Fineberg, E., Evans, D. G., Izatt, L., Eeles, R. A., Adlard, J., et al. (2013). Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 105, 812–822.
Micchelli, C. a and Perrimon, N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–9.
Nagy, P., Kovacs, L., Sandor, G. O. and Juhasz, G. (2016). Stem cell-specific endocytic degradation defects lead to intestinal dysplasia in Drosophila. Dis. Model. Mech.
Zeng, X., Chauhan, C. and Hou, S. X. (2011). Characterization of Midgut Stem Cell– and Enteroblast-Specific Gal4 Lines in Drosophila. 48, 607–611.
Zhao, Z., Oh, S., Li, D., Ni, D., Pirooz, S. D., Lee, J. H., Yang, S., Lee, J. Y., Ghozalli, I., Costanzo, V., et al. (2012). A Dual Role for UVRAG in Maintaining Chromosomal Stability Independent of Autophagy. Dev. Cell 22, 1001–1016.


 (2 votes)
(2 votes)