The rabbit blastocyst modelling (for) vertebrate gastrulation
Posted by Christoph Viebahn, on 5 January 2015
Form and function of animal gastrulation have been longstanding classics accompanying the rise of experimental embryology, and – as if to square the circle in the literal sense – the blastopore of Haeckel’s original ‘gastrea’ stage[1] was soon (and still is) considered analogous to the straight primitive streak of birds and mammals[2-4]. Both forms are capable of fixing the anterior-posterior body axis and of producing the first principal change in cell shape termed epithelio-mesenchymal transition (EMT)[5], which creates the basis for the ‘milieu intérieur’[6] and all internal organs. Current models explaining evolutionary steps between these divergent forms include intermediate morphologies in non-avian sauropsida (such as turtles, lizards and snakes) with a combination of both a small blastopore and a broad blastoporal plate[7] and lead to the proposition of a multiple evolutionary appearance of the ‘derived’ primitive streak[8]. However, despite gastrulation’s famous earmark as the nodal event in human life[9], current hypotheses on mechanisms leading to different gastrulation forms still call for evidence of cellular activity such as movement and proliferation of neighbouring cells which are extensively described in non-amniotes such as Xenopus and Zebrafish[10, 11].
As our lab has a longstanding interest in mammalian body axis specification and formation we recently extended our live cell marking using DiI[12] (s. Figure 1) and multiphoton microscopy of DAPI-stained whole rabbit blastocysts[13]. We modified pre-gastrulation cell movements by inhibiting the Rho-kinase ROCK, a downstream effector of the PCP pathway controlling actin-dependent directed cell motility[14].
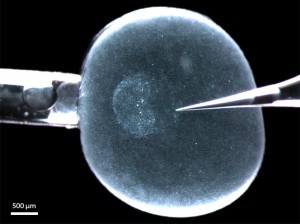
Figure 1: A living rabbit blastocyst (6 d.p.c.) with its zona pellucida equivalent still intact and mounted in a microinjection set-up for depositing DiI label into the (perivitelline) space between epiblast and zona pellucida (s. [12]). The anterior border of the embryonic disc (the anterior marginal crescent) is on the left (on the side of the holding pipette). The glass needle (right) touches the posterior pole, where the primitive streak will appear within the next 6 hours.
As published online 16 December 2014 in DEVELOPMENT (http://www.ncbi.nlm.nih.gov/pubmed/25516971) [15], our time-lapse videos show that cell movements can be disturbed specifically in the future primitive streak forming area: directed intercalation of elongated cells towards the future primitive streak as well as oriented cell divisions are severely disturbed. Intriguingly, expression analysis of several genes involved in primitive streak specification and formation as well as high-resolution morphology revealed different grades of primitive streak deformation in a dose-dependent manner; moreover, abnormal expression domains appeared to mirror gastrula forms known from a variety of vertebrates including putatively ancient forms seen in non-avian amniotes [8].
Implications of this work for the evolution of gastrulation are manifold: a quite compelling scenario for the evolution of vertebrate gastrulation suggests a shift of the circular mesoderm forming domain to the posterior pole to be ‘driven’ by the pressure of an increasing yolk mass; it also includes a further evolutionary step involving narrowing and elongation of the posterior domain into a blastoporal plate or a primitive streak[16]. Our results add some cellular ingredients to this scenario and support a model developed for the evolution of the chick primitive streak, which had brought a temporal shift of PCP-driven processes prior to gastrulation into the picture[17]. The experimentally altered migratory behaviour of neighbouring cells indeed suggests that a step-wise spatiotemporal adjustment of medio-lateral cell intercalation could have led to the transformation of the ancient circular mesoderm forming domain into a ‘precociously’ elongated midline domain, which then turns into a blastoporal plate and/or into a primitive streak. Surprisingly, the mammalian embryo proves to be more flexible than the avian embryo when it comes to ‘mimicking’ different vertebrate gastrula forms such the amphibian blastopore or the teleost embryonic shield.
Further studies could put the rabbit model(ling) to the test by analysing other mammals with a flat embryonic disc such as marsupials for basal mammals (e.g. the tammar wallaby[18]) or chiroptera for derived mammals (e.g. Carollia[19]), and, ideally, in non-human primates. The rise of reptilian model organisms[8], on the other hand, suggests that some mechanisms of the putative intermediate evolutionary step could soon be analyzed directly, also. Apart from this, the highly reproducible and relatively simple experiments on lagomorph blastocysts together with emerging molecular tools for the rabbit (s. [20]) suggest that peri-gastrulation events in mammals will be amenable to analysis of (1) further components guaranteeing directional movements during primitive streak formation, (2) effectors critically dependent on primitive streak formation (and EMT) events, and (3) the emerging PCP orientation prior to directional cell movements, as these herald the most important time of our lives.
Viktoria Stankova, Nikoloz Tsikolia, and Christoph Viebahn
Institute of Anatomy and Embryology, University Medical Centre, University of Göttingen, Germany
References:
1. Haeckel E (1874) Gastreatheorie. Jen Zeitschr Naturwiss 8
2. Kollmann J (1886) “Gastrulasitzung” der 59. Versammlung deutscher Naturforscher und Ärzte zu Berlin. Anat Anz 1: 281-294
3. Pasteels J (1940) Un apercu comparitif de la gastrulation chez les Chorde´s. Biol Rev Camb Philos Soc 15: 59–106
4. Stern CD (2004) Gastrulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA
5. Yang, J., & Weinberg, R. (2008). Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis Developmental Cell, 14 (6), 818-829 DOI: 10.1016/j.devcel.2008.05.009
6. Bernard C (1859) Leçons sur les propriétés physiologiques et les altérations pathologiques des liquides de l`organisme. Paris, Baillière.
7. Coolen, M., Nicolle, D., Plouhinec, J., Gombault, A., Sauka-Spengler, T., Menuet, A., Pieau, C., & Mazan, S. (2008). Molecular Characterization of the Gastrula in the Turtle Emys orbicularis: An Evolutionary Perspective on Gastrulation PLoS ONE, 3 (7) DOI: 10.1371/journal.pone.0002676
8. Bertocchini, F., Alev, C., Nakaya, Y., & Sheng, G. (2013). A little winning streak: The reptilian-eye view of gastrulation in birds Development, Growth & Differentiation, 55 (1), 52-59 DOI: 10.1111/dgd.12014
9. Wolpert L (1992) It’s not birth, marriage or death, which is the most important time in your life, but gastrulation. Quoted by Stern CD and Ingham PW in Development Suppl 1992: I
10. Keller, R. (2002). Shaping the Vertebrate Body Plan by Polarized Embryonic Cell Movements Science, 298 (5600), 1950-1954 DOI: 10.1126/science.1079478
11. Tada, M., & Heisenberg, C. (2012). Convergent extension: using collective cell migration and cell intercalation to shape embryos Development, 139 (21), 3897-3904 DOI: 10.1242/dev.073007
12. Viebahn C, Stortz C, Mitchell SA, & Blum M (2002). Low proliferative and high migratory activity in the area of Brachyury expressing mesoderm progenitor cells in the gastrulating rabbit embryo. Development (Cambridge, England), 129 (10), 2355-65 PMID: 11973268
13. Halacheva, V., Fuchs, M., Dönitz, J., Reupke, T., Püschel, B., & Viebahn, C. (2011). Planar cell movements and oriented cell division during early primitive streak formation in the mammalian embryo Developmental Dynamics, 240 (8), 1905-1916 DOI: 10.1002/dvdy.22687
14. Habas, R., Kato, Y., & He, X. (2001). Wnt/Frizzled Activation of Rho Regulates Vertebrate Gastrulation and Requires a Novel Formin Homology Protein Daam1 Cell, 107 (7), 843-854 DOI: 10.1016/S0092-8674(01)00614-6
15. Stankova, V., Tsikolia, N., & Viebahn, C. (2015). Rho kinase activity controls directional cell movements during primitive streak formation in the rabbit embryo Development, 142 (1), 92-98 DOI: 10.1242/dev.111583
16. Arendt, D., & Nübler-Jung, K. (1999). Rearranging gastrulation in the name of yolk: evolution of gastrulation in yolk-rich amniote eggs Mechanisms of Development, 81 (1-2), 3-22 DOI: 10.1016/S0925-4773(98)00226-3
17. Voiculescu, O., Bertocchini, F., Wolpert, L., Keller, R., & Stern, C. (2007). The amniote primitive streak is defined by epithelial cell intercalation before gastrulation Nature, 449 (7165), 1049-1052 DOI: 10.1038/nature06211
18. Renfree, M. (2010). Review: Marsupials: Placental Mammals with a Difference Placenta, 31 DOI: 10.1016/j.placenta.2009.12.023
19. Cretekos CJ, Weatherbee SD, Chen CH, Badwaik NK, Niswander L, Behringer RR, & Rasweiler JJ 4th (2005). Embryonic staging system for the short-tailed fruit bat, Carollia perspicillata, a model organism for the mammalian order Chiroptera, based upon timed pregnancies in captive-bred animals. Developmental dynamics, 233 (3), 721-38 PMID: 15861401
20. Osteil, P., Tapponnier, Y., Markossian, S., Godet, M., Schmaltz-Panneau, B., Jouneau, L., Cabau, C., Joly, T., Blachere, T., Gocza, E., Bernat, A., Yerle, M., Acloque, H., Hidot, S., Bosze, Z., Duranthon, V., Savatier, P., & Afanassieff, M. (2013). Induced pluripotent stem cells derived from rabbits exhibit some characteristics of naive pluripotency Biology Open, 2 (6), 613-628 DOI: 10.1242/bio.20134242


 (3 votes)
(3 votes)
I would really like to work with rabbit embryos, how difficult is it to get these embryos and how many do you get per female?