A day in the life of a modern Lernaean Hydra…
Posted by Eleni Chrysostomou, on 3 July 2017
I am Eleni Chrysostomou, a PhD student in Uri Frank‘s lab at the National University of Ireland, Galway. The Frank lab’s general interest is development and regeneration, stem and germ cell biology, neural fate commitment, and the chromatin biology underlying these processes. The focus of my project is the roles of SoxB transcription factors (TFs) during nervous system development and regeneration. More specifically, my hypothesis is that SoxB TFs are expressed sequentially in the neural lineage and play a role in neural progenitor cells (NPCs) migration from the body column to the site of injury to re-establish and regenerate the missing head region. The work is mostly done in an in vivo context utilizing transgenic animals, as well as various molecular techniques.
According to the Greek mythology, one of Hercules’ labours was to kill the sea monster Lernaean hydra. What he didn’t know was that every time he decapitated one of the monster’s heads, it would grow back in triplicate! But how is that even remotely related to stem cell biology…
With that said, meet our animal model Hydractinia. Hydractinia, a marine colonial hydrozoan can be described as a great representative of the Cnidaria phylum and an excellent animal model to study cell and developmental biology, as its utility let to the assembly of the very first concepts and terms in biology, including the characterization of stem cells (Weismann, 1883).
The stem cells founded in Hydractinia (aka interstitial cells: i-cells) remain collectively pluripotent throughout the organism’s life and they express germ line markers such as Nanos, Vasa and Piwi (Bradshaw et al., 2015; Plickert et al., 2012). Hydractinia can be easily cultured and genetically manipulated in the laboratory without any ethical restrictions, and its application is suitable in various disciplines.
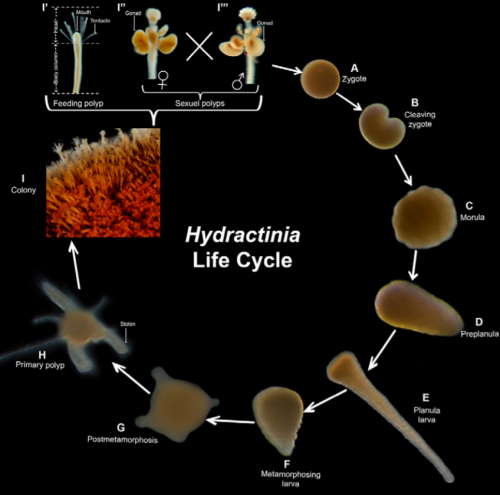
Hydractinia has a relatively simple life cycle. Following fertilization, the embryonic development lasts 36-48 hours and upon induced metamorphosis the primary polyp is asexually reproduced to give rise to the new colony (Figure 1). The resulting members of an individual colony share a gastrovascular space, nervous system and migratory stem cells by maintaining tissue continuity (Gahan et al., 2016)
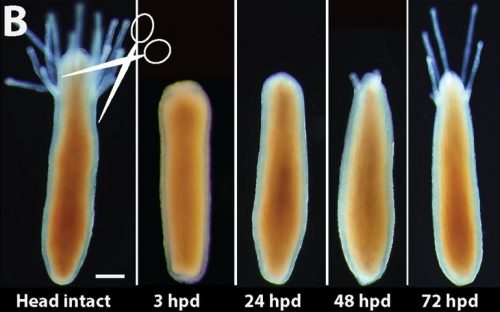
What really makes this animal so intriguing is its regenerative abilities. Like the mythical creature Lernaea, upon decapitation the animal is able to regenerate a fully functional and complete head just within three days (Figure 2).
What do we do with our “non-mainstream” animal model…
The Frank Lab is based in the National University of Ireland Galway and it is composed of post-graduate students and Post-docs with each of us exploring a different aspect of the biology of these animals in order to answer fundamental questions spanning from developmental biology to epigenetics.
Some of the projects that are currently running in our lab are: whole body regeneration from small tissue fragments (Hakima Flici – Post-Doc), the role of Piwi genes in development and regeneration (Emma McMahon – PhD student), histone variants in Hydractinia (Anna Torok – PhD student), sexual commitment (Timothy Dubuc – Post-Doc).
A typical day in our lab is anything but ordinary. We start the day by spawning the animals and collect the embryos for injection. As the time window for injection is quite narrow – only half an hour before they start dividing – you don’t have much of a choice but to wake up and run downstairs to the manipulation room. After the running and once injection is done, you can breathe for a bit but not for long. The animals need to be fed after all that effort given to the spawning. Generally, there is not much time to lay back and enjoy a nice cup of coffee – not a tea person – but we still love it (that’s why we are in research I guess). Once the feeding is done, you can go back to the lab and see what’s the plan for the day and continue with the experiments that you left from last night – most of the protocols have overnight incubations or you were just too tired and hungry to keep working, lets be honest. And before you realize it it’s already late in the afternoon! The working hours in Ireland can be a bit tricky during the summer time (not really summer, but oh well) as the day light can last until 10-11 o’clock in the night and you have no sense of the actual time! The supervisors are happy during that period…
Even though working with a not so popular animal model can be a bit demanding, it gives us the opportunity to study developmental aspects not feasible in other animal models. Although structurally simple, Hydractinia encompasses a complex gene repertoire that is highly conserved to their sister branch bilaterians. Not many organisms are able to regenerate body parts and especially their heads. This pioneering model for stem cell biology gives as a highly valuable advantage to gain insights on why other animals including humans have limited or zero abilities to regenerate missing parts of their bodies.

References
Bradshaw, B., Thompson, K., Frank, U. (2015) Distinct mechanisms underlie oral vs aboral regeneration in the cnidarian Hydractinia echinata. Elife, 4:e05506.
Flici, H., Schnitzler, C.E., Millane, R.C., Govinden, G., Houlihan, A., Boomkamp, S.D., Shen, S., Baxevanis, A.D., Frank, U. (2017) An evolutionarily conserved SoxB-Hdac2 crosstalk regulates neurogenesis in a Cnidarian. Cell Rep, 18: 1395-1409.
Plickert, G., Frank, U., Muller, W.A. (2012) Hydractinia, a pioneering model for stem cell biology and reprogramming somatic cells in pluripotency. Int J Dev Biol, 56:519-534.
Weismann, A. (1883). The origin of the sexual cells in hydromedusae. Jena. Gustav Fischer
Gahan, J.M., Bradshaw, B., Flici, H., Frank, U. (2016) The interstitial stem cells in Hydractinia and their role in regeneration. Curr Op Gen & Dev, 40:65-73.


 (6 votes)
(6 votes)