In Development this week (Vol. 138, Issue 7)
Posted by Seema Grewal, on 8 March 2011
Here are the research highlights from the current issue of Development:
A breath of fresh air: miRNAs regulate lung development
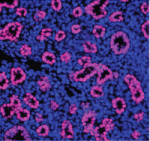
Throughout development, a proper balance between the proliferation and differentiation of progenitor cells is essential but the gene regulatory networks that control this balance are only partly understood. Here, Edward Morrisey and colleagues report that miR302/367 (a microRNA cluster) regulates the behaviour of endoderm progenitor cells during mouse lung development (see p. 1235). MicroRNAs (short RNA molecules that silence complementary target mRNA sequences) are expressed in clusters from a single primary transcript. The researchers show that, in early lung endoderm, the miR302/367 cluster is a target of the transcription factor Gata6, which is known to regulate lung endoderm progenitor differentiation and proliferation. Increased or decreased miR302/367 expression, they report, alters the balance of lung endoderm progenitor differentiation and proliferation in part through regulation of the tumour suppressor Rbl2 and the cell-cycle regulator Cdkn1a. Notably, altered miR302/367 expression also disrupts apical-basal polarity of endoderm progenitor cells. Thus, the researchers conclude, miR302/367 directs mouse lung development by regulating multiple aspects of lung endoderm progenitor cell behaviour.
Canonical Wnt9b signals size the kidney
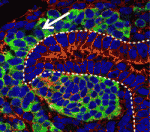
During kidney development, the balance between nephron progenitor cell differentiation and proliferation determines the final number of nephrons and the ability of the kidney to function properly. One current model proposes that Wnt9b/β-catenin signalling induces differentiation in a subset of the progenitors, but that repression of this signal by the transcription factor Six2 is required for renewal of the remaining progenitors. On p. 1247, Thomas Carroll and colleagues challenge this model by showing that Wnt9b/β-catenin signalling is active in both differentiating and renewing progenitor cells in the developing mouse kidney. Moreover, rather than inhibiting Wnt9b signalling in the renewing cells, Six2 acts cooperatively with Wnt9b to elicit progenitor cell expansion. By contrast, in those progenitor cells where Six2 activity is low, Wnt9b/β-catenin signalling induces differentiation. Thus, the researchers propose, the response of progenitor cells to Wnt9b/β-catenin signalling depends on the cellular environment in which the signal is received, and canonical Wnt9b signalling is able to regulate both progenitor cell expansion and differentiation in the developing kidney.
Endoderm specifies germline niche
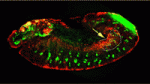
Interactions between tissue-specific stem cells and their local niche are vital for stem cell self-renewal and differentiation. But how are niches established? On p. 1259, Tishina Okegbe and Stephen DiNardo provide new insights into testis stem cell niche development in Drosophila. The stem cells in the fly testis, which sustain spermatogenesis throughout life, are clustered around a group of somatic cells (hub cells) that serves as a niche. The researchers confirm a previous report that Notch signalling is necessary for specification of mesoderm-derived somatic gonadal precursor cells to the hub cell fate but, unexpectedly, show that the endoderm adjacent to the developing testis supplies the Notch-activating ligand Delta. They also report that niche cell specification occurs earlier than anticipated, well before the expression of known niche cell markers. Given that mammalian primordial germ cells also pass through endoderm on their way to the genital ridge, the researchers suggest that Delta-Notch signalling by the endoderm could be a conserved mechanism for specification of the germline niche.
Glia shape up sensory neurons
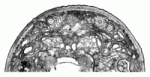
Neuronal receptive endings (for example, sensory protrusions) are remodelled by experience; but how do they acquire their new shape? To address this question, Shai Shaham and co-workers have been studying the remodelling of sensory neuron receptive endings that occurs in C. elegans during dauer (a developmental state induced by environmental stressors). They now report that glial cells delimit this remodelling in response to external cues (see p. 1371). Nematodes have two AWC (olfactory) neurons, each of which is enveloped by an amphid sheath (AMsh) glial cell. The researchers show that AMsh glial remodelling is required for the shape changes in AWC sensory neuron receptive endings in dauers, and that glial remodelling requires the AFF-1 fusogen, the transcription factor TTX-1 and probably the VEGFR-related protein VER-1. The expression of ver-1, they report, requires binding of TTX-1 to ver-1 regulatory sequences, and is induced by dauer entry. Together, these results suggest that stimulus-induced changes in glial compartment size spatially constrain the growth of neuronal receptive endings.
Keeping an Eya1 on lung cell polarity
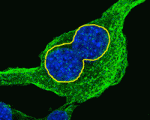
To function correctly, the epithelial cells that line the tubes and air sacs of mammalian lungs need to be polarised. Little is known about the mechanisms that control cell polarity in the lung epithelium but now, on p. 1395, David Warburton and co-workers implicate the protein phosphatase Eya1 in cell polarity control in the mouse distal embryonic lung epithelium, which represents the epithelial progenitor pool. The researchers show that distal embryonic lung epithelium is polarised with characteristic perpendicular cell divisions. They report that several spindle orientation-regulatory proteins and the cell fate determinant Numb are asymmetrically localised in distal embryonic lung epithelium. Furthermore, interfering with the function of these proteins in vitro randomises spindle orientation and alters cell fate. Importantly, the researchers show that interfering with Eya1 function in vivo or in vitro results in defective epithelial cell polarity and mitotic spindle orientation, disrupts Numb segregation, and inactivates Notch signalling, thereby establishing Eya1 as a crucial regulator of the complex behaviour of distal embryonic lung epithelium.
Heartfelt Slit/Robo signals
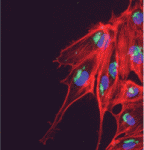
During vertebrate heart development, myocardial and endocardial precursors migrate towards the embryonic midline where they fuse into a linear heart tube. Now, Jason Fish, Stephanie Woo and colleagues report that a Slit/miR-218/Robo signalling pathway regulates heart tube assembly in zebrafish (see p. 1409). Members of the Slit family of secreted ligands interact with Roundabout (Robo) receptors to provide guidance cues during the development of several organs; development is also regulated by microRNAs (miRNAs) that can fine-tune the expression of developmentally important genes. The researchers show that the conserved miRNA miR-218 is intronically encoded in slit2 and slit3, and that it suppresses Robo1 and Robo2 expression. Further analyses indicate that Slit2, Robo1 and miR-218 are required for the formation of the zebrafish heart tube and that these factors act, in part, by modulating Vegf signalling. These findings reveal a novel signalling pathway for vertebrate heart tube formation and suggest a new paradigm of receptor/ligand regulation in which a ligand-encoded microRNA regulates the expression of its own receptor.


 (No Ratings Yet)
(No Ratings Yet)