Scaling the Fish: An L.A. Story
Posted by Jeff Rasmussen, on 18 October 2018
Jeff Rasmussen tells the story behind his recent paper from the Sagasti Lab in Dev Cell.
This project began as an extension of my earlier postdoc work in Alvaro Sagasti’s lab investigating removal of axon debris following skin injuries in the larval zebrafish [1] and led me into scientific territory that I never anticipated. It is a story that would not have happened without open-mindedness, encouragement and—most importantly—help from colleagues in the fish community.
Sensory axon endings profusely innervate the skin, and skin injuries trigger axon degeneration. We previously discovered that keratinocytes are the primary phagocyte for degenerating axons in larval skin. But what cells eat axons that degenerate in the more complex adult skin?
In order to answer this question, I first needed a way to visualize sensory axons in adults. Most work in zebrafish has focused on the larval system, so markers for adults have lagged behind. Luckily, I came across a transgenic line made by a previous graduate student in Alvaro’s lab [2] that showed bright and specific expression of sensory axons in adults. The pattern of adult skin innervation revealed by this line caused this project (and my career) to take a twist.
A Striking Axon Pattern
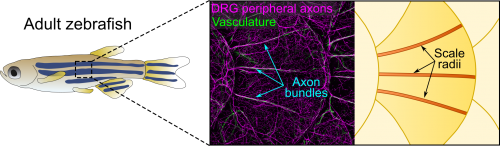
Axons of Rohon-Beard neurons innervate most of the larval fish skin. Rohon-Beard axons in the skin rarely bundle (fasciculate). By contrast, this transgenic line revealed that axons of dorsal root ganglion (DRG) neurons, which innervate adult skin, were frequently bundled. More surprisingly, these bundles were evenly spaced across the surface of scales, which underlie the adult epidermis (Figure 1). Alvaro whole-heartedly encouraged me to dig deeper into what created these evenly spaced bundles, despite his lab having no experience working with post-embryonic stages.
Thi’s Developmental Studies
Thi Vo, an undergraduate researcher in Alvaro’s lab, took on the challenge of analyzing post-embryonic development. Because many Rohon-Beard neurons die after only a few days of development, we expected that DRG axons would innervate the epidermis shortly thereafter. However, by staining isolated scales to visualize axons, Thi found that the axon bundles only appeared during late juvenile stages. So Thi next analyzed mutants lacking DRG neurons and found that the bundles never formed, proving that they were indeed DRG axons.
Thi also stained scales with phalloidin to visualize cell morphology and found that a set of elongated cells apparently presaged the path of the axon bundles. What could these cells be?
Lindsey’s Schwann cells
We initially hypothesized that the elongated cells were Schwann cells, the glia of the peripheral nervous system. Schwann cells are neural-crest derived, and Shannon Fisher’s group recently made a neural crest lineage reporter [3], which we learned was growing in Gage Crump’s lab at USC. To determine if the line labeled Schwann cells in adults, I made a trip across town to visit Lindsey Barske, a postdoc in the Crump lab, who was working with the neural crest reporters. One of Lindsey’s lines labeled Schwann cells coating the axon bundles, showing that they are nerves and giving us a key reagent to visualize their development. But when Thi and I analyzed the timing of Schwann cell migration, they appeared too late to pioneer the path of the DRG bundles, ruling out this hypothesis.
Michael’s Vessels
After hearing about our work at a Southern California Zebrafish Meeting, Michael Harrison, a postdoc in Ellen Lien’s lab working on heart and vascular development, suggested we analyze several of his chemokine mutants. Thi and I took a bus trip down Sunset Blvd to CHLA to collect scales from these mutants. Although this effort was ultimately fruitless, as luck would have it, Michael’s mutants expressed multiple transgenic reporters. One of these transgenes was fli1a:EGFP (a vascular reporter made by Brant Weinstein’s group) [4], which revealed that the axon bundles tightly associated with blood vessels (Figure 1). This was unexpected because we had initially ruled out a vascular contribution based on analysis of a vessel reporter not as broadly expressed as fli1a.
Intriguingly, axons and vessels also tightly associate in mammalian skin and axons promote vascular remodeling and arterial differentiation in mouse [5]. Were blood vessels the elongated cells that arose early in scale development? No, since we found that blood vessels only appeared along mature scales, once animals reached adulthood. What about the converse: did axons pattern the vessels? By again analyzing mutants lacking sensory neurons, we found that blood vessels appeared normal. Thus, in contrast to mammals, skin nerves and vasculature are independently patterned in fish. This was an important finding but, once again, the identity of the pioneering cells remained elusive.
Sandeep’s Osteoblasts
The scale surface is made from bone and contains a number of striking patterns. Remarkably, we noticed that the axon bundles and vessels aligned with scale radii, grooves in the bone that radiate from the scale center (Figure 1). Could osteoblasts, bone-forming cells, or osteoclasts, bone-degrading cells, guide axons and vessels? We first examined osteoclasts but found no evidence that they form the radii. Next, I made another crosstown trip to the Crump lab—this time with the help of Sandeep Paul—to look at osteoblast reporters. Sandeep’s lines showed that osteoblasts line mature radii, as suggested by pioneering ultrastructural studies [6, 7]. Imaging osteoblasts early in scale development revealed that they create the radial paths by polarized migration.
To test if osteoblasts promote skin innervation during regeneration, we used an inducible osteoblast ablation line made by Ken Poss’ group [8] and found that blocking scale regeneration by osteoblast ablation resulted in a reduction of axon density. To test if scale development similarly promoted innervation, we analyzed mutants that prevent scale development [9, 10]—provided as part of a “scale care package” by Matt Harris’ lab. These mutants had reduced skin innervation and vascularization, showing that scales are also required during ontogeny.

Full Scale Ahead
Although I never planned to work on scales as a model system, I am really excited about the potential for these mini-organs to reveal the cellular and molecular basis for cell type patterning during skin development and repair—questions that I will be pursuing in my newly formed research group at the University of Washington. Scales are evolutionarily related to other types of specialized skin appendages like feathers and hair. Thus, studies of fish scales may reveal general mechanisms for coupling organ maturation and growth to skin patterning. Scales may also yield insights into bone-nerve interactions that occur in diverse tissues, like antlers, teeth and long bones. An increasing number of genetic tools, together with advances in live-cell imaging of post-embryonic stages (Figure 2), suggest the future is bright for a resurgence of scales as a model system.
References
[1] Rasmussen JP, Sack GS, Martin SM, Sagasti A. Vertebrate epidermal cells are broad-specificity phagocytes that clear sensory axon debris. J Neurosci. 2015;35(2):559–70.
[2] Palanca AMS, Lee SL, Yee LE, Joe-Wong C, Trinh LA, Hiroyasu E, et al. New transgenic reporters identify somatosensory neuron subtypes in larval zebrafish. Dev Neurobiol. 2013;73(2):152–167.
[3] Kague E, Gallagher M, Burke S, Parsons M, Franz-Odendaal T, Fisher S. Skeletogenic fate of zebrafish cranial and trunk neural crest. PLoS One. 2012;7(11):e47394.
[4] Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248(2):307–18.
[5] Mukouyama Ys, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109(6):693–705.
[6] Waterman RE. Fine structure of scale development in the teleost, Brachydanio rerio. Anat Rec. 1970;168(3):361–379.
[7] Sire JY, Allizard F, Babiar O, Bourguignon J, Quilhac A. Scale development in zebrafish (Danio rerio). J Anat. 1997;190 ( Pt 4):545–561.
[8] Singh SP, Holdway JE, Poss KD. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell. 2012;22(4):879–886.
[9] Harris MP, Rohner N, Schwarz H, Perathoner S, Konstantinidis P, Nusslein-Volhard C. Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates. PLoS Genet. 2008;4(10):e1000206.
[10] Rohner N, Bercsenyi M, Orban L, Kolanczyk ME, Linke D, Brand M, et al. Duplication of fgfr1 permits Fgf signaling to serve as a target for selection during domestication. Curr Biol. 2009;19(19):1642–1647.


 (1 votes)
(1 votes)