Rare metabolic disorder replicated in rice fish
Posted by the Node, on 9 June 2021
A Press Release from Development
A mutation from a human patient with a rare metabolic disorder has been replicated in the Japanese rice fish. Researchers from the Centre for Organismal Studies Heidelberg, Germany, have developed a fish model to study disorders caused by a deficiency in the process of adding sugar molecules to proteins. These findings, published in the journal Development, provide a system to study the causes of complex metabolic disorders in humans.
Human cells are kept healthy by the activity of millions of proteins. These proteins are modified in different ways, such as by adding sugar molecules to them, which can be crucial for them to function properly. Given this importance, defects in the sugar-adding process are often lethal at the very early stages of development. In rare cases, however, patients can develop sugar-adding deficiencies that result in a range of metabolic diseases, known collectively as ‘congenital disorders of glycosylation’ (CDG). These disorders are caused by defects in the enzymes involved in the sugar-adding process. For example, ALG2-CDG (or CDG-Ii) is a disorder caused by mutations in the ALG2 enzyme, which combines sugar molecules together. ALG2-CDG patients appear unaffected at birth, but later develop problems in different organs, such as the eyes, brain and muscles.
The rarity, variety and complexity of these disorders has made them difficult to study, especially in the context of the whole body. Now, scientists have developed the Japanese rice fish (also known as the medaka) as a model system for studying such disorders. “Fish are particularly good models for these disorders because they develop outside the mother, making them very suitable for studying early embryonic defects,” said Professor Joachim Wittbrodt from the Centre for Organismal Studies, who led the study with Dr Thomas Thumberger. “The medaka is particularly well suited to this type of research, because we can edit the genome with high efficiency and we can utilise genetically identical lines.”
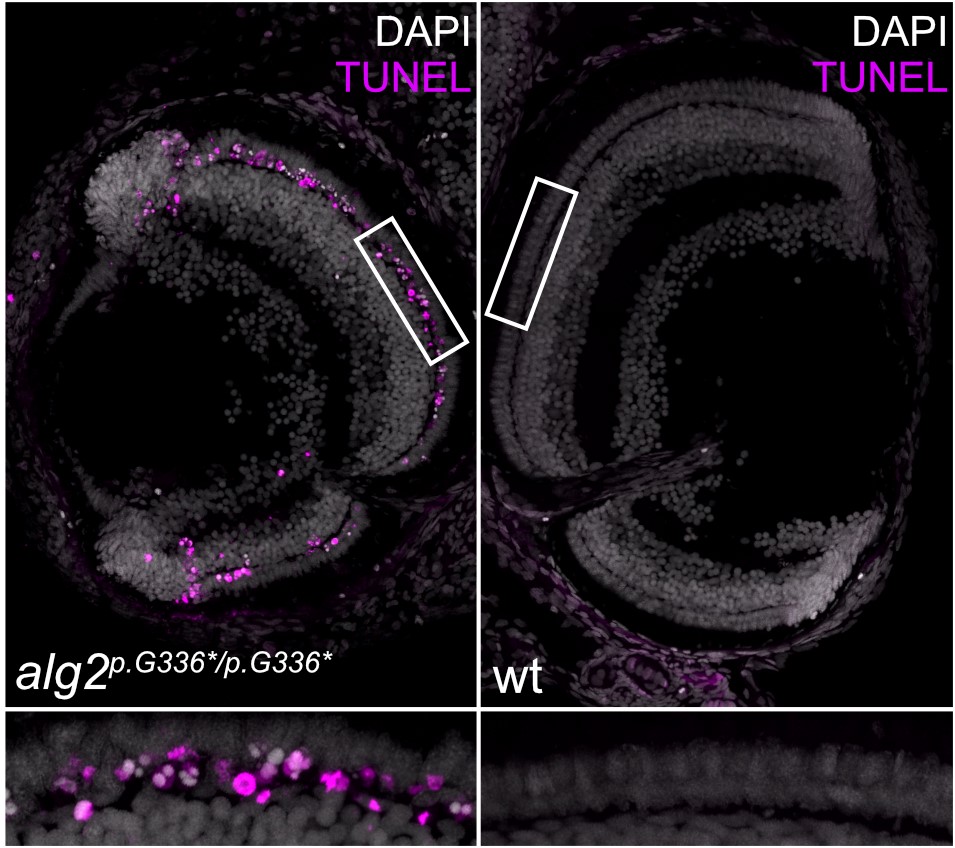
The team used CRISPR/Cas9 genome editing to introduce mutations in the medaka’s alg2 gene, in the same region where mutations had been found in a patient with ALG2-CDG. The scientists found that many of the symptoms of the patient, such as neuronal problems, were replicated in the fish. “We basically discovered a large fraction of the symptoms that had been described in the patient. Unlike studies of cells in a dish, the fish model provides the full spectrum of different cell types in an organism, which produced some unexpected results. For example, even though all the cells lack ALG2 enzymatic activity, only some cells respond, while their neighbours do not. In the fish eye, the cone (colour-sensing) cells are unaffected, whereas rod cells (which are required for vision in low light) form initially, but are then eventually lost. This defect, known as retinitis pigmentosa, is a symptom of many patients with congenital disorders of glycosylation,” explained Professor Wittbrodt. “We want to identify the proteins that require ALG2 in rod cells to understand their involvement in maintaining rod-cell function,” he added.
Importantly, these defects could be prevented by supplying fully-functional Alg2 to the fish eggs. Moving forward, the researchers plan to use this animal model to study the effects of human ALG2 mutations further. Professor Wittbrodt said, “the fact that this disorder can be efficiently rescued opens the door for understanding how different mutations in ALG2 affect its function. We especially want to study the cell type-specific responses in the context of a whole organism.”
Gücüm, S., Sakson, R., Hoffmann, M., Grote, V., Becker, C., Pakari, K., Beedgen, L., Thiel, C., Rapp, E., Ruppert, T., Thumberger, T., Wittbrodt, J. (2021) A patient-based medaka alg2 mutant as a model for hypo-N-glycosylation. Development, 148, dev199385. doi:10.1242/dev.199385


 (No Ratings Yet)
(No Ratings Yet)