An interview with Scott Gilbert
Posted by Helen Zenner, on 1 March 2023
Scott Gilbert is Emeritus Professor of Biology at Swarthmore College and the University of Helsinki. He literally wrote the book on developmental biology! With the 13th edition of ‘Developmental Biology’ about to be published, we took the opportunity to find out more about the story behind the textbook, to discuss Scott’s research career and his social and political commentaries in relation to developmental biology.

Let’s start at the beginning, when did you first become interested in biology?
I think that everyone is initially interested in biology; but for some odd reason, most people aren’t able to keep that interest. Most young people love dinosaurs, turtles, and horses, but they leave those interests behind. I was always fascinated by nature. My summer days were spent in the woods and fields of northern Pennsylvania, finding salamanders, frogs, and butterflies. My scientific career peaked when I was 12 years old. I had caught a five-legged frog, and word of this somehow got to Leonard Lyons, the syndicated gossip columnist for the New York Post. So, I was mentioned in his New York Post column, alongside Josephine Baker, Leo Szilard, and Pablo Casals. I don’t expect to ever reach this pinnacle again. I was also fortunate to have met Dr Jean Cook, a Black haematologist at the Albert Einstein College of Medicine, who gave me my first research experience while in high school. He was fascinated by the ways that life could have originated in the early atmosphere of the planet. In trying to repeat the Urey-Miller experiment, I nearly fried myself, trying to splice wires together while they were still plugged into the sparking coil – a learning experience (and I did get to identify aminoacrylonitriles). In addition to teaching me about erythrocyte development and how to do column chromatography, he also taught me to appreciate and enjoy the music of Julian Bream.
You went on to study biology with religion at university, what prompted you to choose that combination?
I always thought I was going to be a biology major, but in college, I also became fascinated by the study of religion. I came to the conclusion that the ecology movement needed an alliance of science and religion, and that the two areas were actually related. I see ‘Wonder’ as the prime experience that the mind has with the world. But wonder has a short half-life and ‘decays’ into curiosity (I wonder) and awe (the wonder of the world.) Curiosity gives rise to science and philosophy; awe gives rise to reverence and the religious attitude. So, science and religion are both the ‘grandchildren’ of wonder. Moreover, science and religion have a vested interest in keeping those sources of wonder alive. I don’t care whether a person goes into the environmental movement to preserve biodiversity, or to be a proper steward of God’s creation. They’re both motivations for preserving natural wonder, which I feel is critical, among other things, for preserving science.
In 2016, I was asked to give a lecture on developmental biology to His Holiness, the Dalai Lama. After my talk, the Dalai Lama said that he enjoyed the talk, but that the questions of interest to him are: How does the reptilian brain become the mammalian brain, and how does the mammalian brain become the human brain? So, we have a religious leader who not only accepts evolution, but who wants to know the evolutionary developmental mechanisms of neocortex formation!
Do you think that it is important for scientists to comment on social and political issues that are related to their work? Have you faced any criticism for your papers and commentary?
As embryologists we have to deal with issues of sexism, racism, and abortion. If we’re working in evo-devo, we’ll come across creationists. Saying nothing about these social issues is itself a political position. If you have expertise in these fields yet don’t combat publicly stated falsehoods, then you’re basically saying that the status quo is acceptable. Most of my social commentary has been against people misusing developmental biology to say that science supports white supremacist or male supremacist arguments. I think it’s because I love developmental biology and I don’t want to see my beloved being smeared! Having a background in history of biology has helped me enormously in addressing these issues. I’ve written about how scientists in the 1800s tried to use human embryology to show that women and Black people were immature, embryonic, forms of the white male. Until very recently, the female was seen as the incomplete development of the male phenotype.
My most recent paper concerns myths that are currently being used in the abortion debate in America. Each of these myths is represented as science, but none of them has much to do with facts from developmental biology. These myths portray fertilisation as ensoulment, and depict the woman as a passive entity. I also show that different groups of biologists claim different embryonic stages to be the start of personhood (admitting that ‘personhood’ is not really a scientific concept), and that the notion that all biologists believe that personhood begins at fertilisation is ideology, not science. Some biologists say it begins at fertilisation when you get your genome. Other biologists say it begins at gastrulation, where you become an individual and can’t form twins or triplets anymore. Other biologists have claimed personhood begins when you get your electroencephalogram (EEG) pattern, because that’s when the anatomical correlates of consciousness and pain perception form (and we’re willing to say death is the loss of that pattern). Still others say personhood begin around when the fetus becomes viable outside the womb. And some people maintain that personhood begins at birth, when the first breath causes pressure differences that change endothelial gene expression and cardiac morphology, preparing the fetus for life outside the mother. I think the public should be made aware of these different possibilities. I haven’t faced criticism for my views, which probably means they are not well enough known.
Can you give us a brief overview of your research career?
I feel like the surfer that caught the perfect wave. I was at Swarthmore College with a background in developmental biology and history of biology, and that allowed me to catch and ride the waves of developmental biology and the evo-devo wave behind it. As I mentioned, I was an undergraduate at Wesleyan University in Connecticut, where I majored in biology and religion. I did research on the attachment of DNA to the nuclear envelope during sea urchin fertilisation in the laboratory of Tony Infante, and it was published in Nature New Biology. Teaching during the summers at an NSF-sponsored Pre-College Science Center run by Michael Somers convinced me that embryology was the field that most excited me. I pursued my PhD at the Johns Hopkins University in the laboratory of Dr Barbara Migeon, who is most famous for her work in X chromosome inactivation. My project concerned extending amniocentesis to look at the genes of human kidney cells, not just fibroblasts. This was before the days of DNA sequencing, and it meant doing enzyme assays in the cold room. I should mention at this point that I probably hold the record at Hopkins (and maybe elsewhere) for the number of sequential thesis advisors: four. While doing my PhD, I was also able to work on an MA in the history of biology under the direction of Donna Haraway. I did my master’s thesis on how the X chromosome was the bridge between Thomas Hunt Morgan’s embryology and his genetics.
For my first postdoc, I went to the University of Wisconsin to work with Masayasu Nomura, because I thought that E. coli ribosomes would be the best model for looking at the co-transcription of eukaryotic genes. I thought I could prove the Britten-Davidson model using E. coli ribosomes. We published some of the first gene sequences using the Maxam–Gilbert technique with restriction enzymes, polymerases and gel apparatus that we had isolated and made ourselves. Within a few months I had disproven my thesis, but we got some beautiful sequences of the regulatory region for RNA operons! I did a second two-year postdoc in developmental immunology with Robert Auerbach, while my wife was doing her residency in obstetrics and gynaecology. Here, we made monoclonal antibodies against poliovirus, and we discovered the mechanism by which antibodies neutralise the virus.
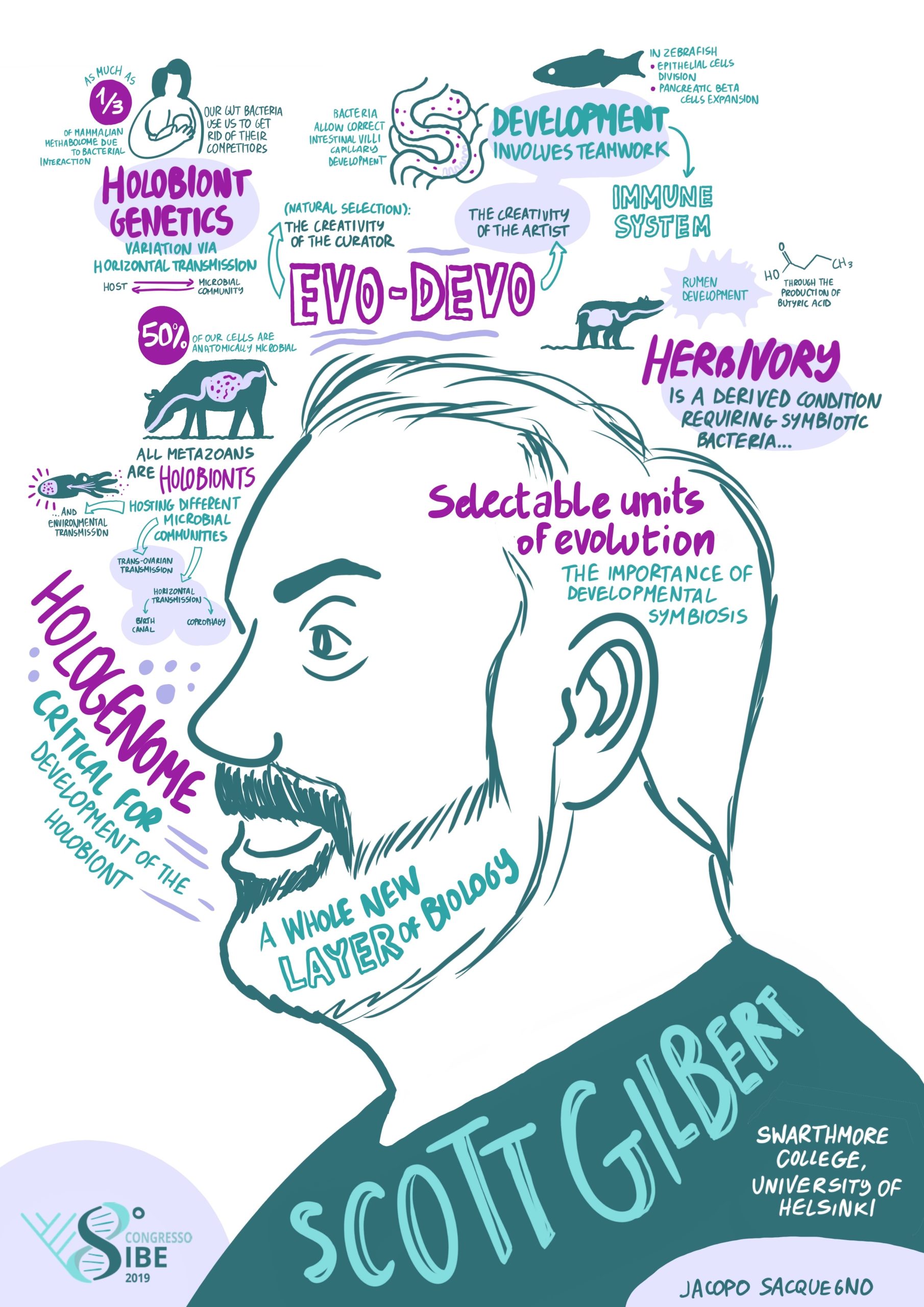
Moving on to the next step, I wanted a career where I could help raise children, have friends, and do other things besides science. But at the same time, I wanted a life that was saturated with science and research. I found this at Swarthmore College, a liberal art college in Pennsylvania. It had one of the best undergraduate biology departments in the country, and there were some phenomenal students coming into Swarthmore. My first paper from Swarthmore came after I heard a lecture on auxin transport given by our plant physiologist, Mark Jacobs. He had an assay for this transport, but he had no way to identify the proteins involved in transferring the hormone from one cell to another. I suggested using monoclonal antibodies (which I knew how to prepare). So, Mark and I published the first paper (in Science) using monoclonal antibodies to study plant development, identifying the location of the auxin transport proteins. I was able to get NSF grants because I was teaching undergraduate students how to do developmental biology. Originally, I continued my work on kidney development, and in 1990, I had the opportunity of spending a sabbatical leave in Finland with Lauri Saxén. Here, I worked on cell-cell communication and branching morphogenesis in mouse kidneys. I thoroughly enjoyed this time in Finland, and I brought back an interest in studying branched organ morphogenesis. Working with Judy Cebra-Thomas, we delineated paracrine factor involvement in the branching of amniote kidneys and lungs. Then I heard at a meeting that Brigid Hogan and Saverio Bellusci were going to work on this problem, and I knew that they could do in a year what would take us five years to do. It was time to get out! During this time, I had been writing about evolutionary developmental biology, so I decided that this would be the context for my next research project. After mooting a number of possibilities, I decided that asking how the turtle got its shell would be a really good project, and we began collaborating with several bone developmental biology laboratories in the Philadelphia area. Many undergrads got their first research experience working on turtle embryos, and grad students working on serious human bone diseases could have some relief working with our turtles. We proposed a paracrine factor hypothesis for the origin of the carapace, and we have evidence that cells migrating the trunk neural crest may actually form the plastron. Many years later, I decided that if I really wanted to get answers to these questions, I had to get into a research laboratory. So, I went half-time at Swarthmore, and received a half-time position at the University of Helsinki, working in the lab of Jukka Jernvall. We’re still working on the turtle project. I no longer have a lab, but Judy Cebra-Thomas has really interesting data that, if confirmed, will give us a great story on how the turtle gets both its carapace and its plastron. Former student Tyler Lyson has been integrating our developmental data into the paleontological record to identify the mechanisms by which turtles may have evolved their shells. The turtle has gone from being an anomaly to being a prime example of how developmental biology and palaeontology can interact to construct an account of how organisms evolved. I’m presently pursuing work on holobiont development (discussed below).
Working at a liberal arts college such as Swarthmore, you’re expected to do research because it’s the best way of teaching your undergrads. Of course, you go a bit slower because you’re teaching methods and attitudes. (I know how to do an in situ hybridization; my job is to teach the students how to do it; and they do not get it right the first time.) Also, a teacher at a liberal arts college has the ability to work with people outside one’s own department. This has allowed me to publish on the aesthetics of embryology, the roles of puns in science education, the bone that the Bible says created Eve (it wasn’t a rib!), and the use of embryos in the art of Gustav Klimt and Frida Kahlo. My students and I have collaborated to publish papers on Intelligent Design (writing this paper was their final exam), temperature-sensitive holobionts (another final exam), and feminist critiques of biology (which was one of the first papers in the field). When I gave a history of biology course, I saw that the students’ term papers on bioethics would make a fascinating book. Why should I be the sole audience for these investigations? So, with two students as co-editors, we published it. All-in-all, I’ve published nearly 200 papers, many of them concerning the history, philosophy, and social situatedness of biology.
What drew you to the field of evo-devo, and now eco-devo?
My interest in evo-devo comes from my background in the history of embryology. I took a master’s in the history of embryology, and I had directed readings with Bill Coleman on the science of Thomas Huxley, with Camille Limoges on the biology of Sir Richard Owen, and with Donna Haraway on the history of embryology, where we discussed Waddington and others. This meant that I knew the background for evolutionary theory that came out of embryology; I was exapted for evo-devo. I knew about homology and about developmental notions of adaptation. I had read EB Wilson, Frank Lilly, Fritz Müller and Aleksander Kovalevsky. I knew about these people who had a developmental approach to evolutionary biology. In our 1996 paper, John Opitz, Rudolf Raff and I wrote that we can now return to these questions that we had abandoned, that we now have the techniques to go back and look at the notions of homology, look at the notions of heterochrony, look at these notions that we abandoned largely for genetics at the turn of the last century.
So, I was similarly pre-adapted for eco-devo. Again, I knew the history. I knew about the debates between Oscar Hertwig and August Weismann and the examples of developmental plasticity and context-dependent phenotypes. I was encouraged in this area by Donna Haraway, Evelyn Fox Keller and Anne Fausto-Sterling, who were railing against genetic determinism. I found that there was another embryologist who had retired to Swarthmore, NJ (Jack) Berrill. Berrill’s developmental biology books were amazing, and he was adamant that genetics could not explain development. So, I had all these people around me who were talking about environmental regulation of development. Then, in 2001 I had a ‘eureka moment’ when I read the article by Laura Hooper and her colleagues on bacteria controlling gene expression in the mouse intestine. It made me think that this was the missing piece, the influence of microbes on development. They showed that commensal bacteria induced angiogenin-4 production in our intestinal cells, and this secreted protein induced the adjacent mesoderm to become the blood vessels of the gut. This was not only a good thing for the mouse’s life, this protein also helped the microbes by killing Listeria and other competitors. Moreover, mice that lack bacteria are asocial. Their brains were different. In making the mice social, they created the conditions for mouse reproduction, which means not only more mice, but more bacteria.
I’ve become fascinated by the development and evolution of the holobiont, the organised consortium of symbionts. We humans are not only organisms but biomes. We develop with help from other species. How do we receive bacteria and how are they structured in the body? How do they induce the maturation of the gut neurons and the auditory neurons? How do they help mature the immune system, which then patrols our bodies? Does natural selection select ‘teams’ rather than individual players? Are we losing bacteria that are crucial for our normal development? These are new questions for developmental biologists, and they relate directly to environmental concerns.
How did you come to write the first version of ‘Developmental Biology’, which was published in 1985?
The first version was written out of frustration. I had just accepted the position of teaching developmental biology at Swarthmore, and I was frustrated because I had no book that I felt I could teach from. Developmental biology had changed; there were books on ectoderm, endoderm, and mesoderm, with scant mention that genes might be involved. Conversely, there were textbook on RNA, DNA, proteins in development, with only a hint that they somehow formed mesoderm etc. I wanted a view of developmental biology that would integrate classical embryology, cell biology, and molecular biology. Apparently, many other young developmental biologists were thinking the same way and the publishers realised this. So, in 1980, Andy Sinauer, the publisher of ‘Developmental Biology’, was trying to find a person to write such a book. He wanted David Sonneborn to write one, so he went to visit David at University of Wisconsin. According to David, he told Andy that he had a great lab, a great project, and grant money, so he wasn’t going to write such a book. But he said that there was this postdoc down the hall who’s been griping that he doesn’t have a textbook to teach from, and that maybe he should talk with him. So, I got to speak with Andy Sinauer, who suggested that I send him three sample chapters that he would send out for review. The summer before I came to Swarthmore, he gave me the go-ahead to write the book. I later found out that many other people had been thinking about writing textbooks for the same reason I did; but when my textbook came out, there was lateral inhibition. My textbook was similar enough to what they wanted, so they didn’t have to write their own books.
I owe a lot to Andy Sinauer and the fact he was willing to take a chance on me as a postdoc. It was five years before the book first came out. It actually took four years to write, but we had some delays. This ended up being a good thing, because in 1984/85, John Gerhart published his Nieuwkoop center articles, and Katherine Anderson published that maternal mRNA in Drosophila is important for the dorsal ventral axis patterning. The molecularisation of the field was really starting at this time, and I was able to get that into the book.
Another example of when working with a small publishing house was an advantage came in 1997. I was in the cafeteria at my college early in the morning, reading The New York Times. I had a really nasty moment. I don’t know what I yelled, but I immediately ran up to my office, called my editor, Carol Wigg, and asked her if the book manuscript had gone out to the printers yet. She told me that it was in the outbox and would go out that morning. I asked her to grab it and bring it back to her desk. She asked what had happened, and I said they cloned a sheep! We had specifically said in the book that no adult mammal had ever been cloned from a differentiated mammalian cell. Now it had been done; Dolly had arrived. Andy Sinauer called me back a few hours later and told me that I could have one sentence – 120 characters – to replace the sentence we had written earlier. So, when that book came out, about four months later, Dolly was in it!
With the field often moving so quickly, how do you decide what to update in each edition?
I update every chapter in the book (and with my new co-author, Michael Barresi, on board, I have fewer chapters to revise). The small revisions are made piecemeal. Usually, when I read something that I find interesting, I’ll write a paragraph about it and file it away. Then I can go back to it when I’m revising the chapter. Going to meetings is absolutely crucial for revising the chapters, because at meetings, especially at the poster sessions, bars, and meals, I’ll hear about the unpublished work. For instance, I first heard about the SRY gene from Albert de la Chapelle at a meal at a conference. The research hadn’t been published yet. That’s why, as good as Zoom is, it shouldn’t replace scientific meetings. Revising is also much easier now because of the internet. I used to have to go to the college library with my list of journals. I even had a key because I often got there before the librarians! Now, I go to PubMed or Web of Science, and I type in sea urchin fertilisation, chick gastrulation etc. and I’ll find things that I never would have seen by looking in journals.
The new chapters happen when there are big changes in the field. The evo-devo chapter came about from anger and bewilderment! I was at a meeting where some theoretical biologists were claiming that you can discuss evolution mathematically, without knowing about development or even the organism. I felt that this was clearly wrong, and so I put an evolution and development chapter into the book, giving examples of how evolution actually occurs by developmental changes. The ecological developmental chapter was written after a challenge by Cor van der Weele, who criticised my textbook at a meeting, saying that all my examples of developmental plasticity were in the ‘sidelights and speculations’ sections, literally marginalising them. I replied that there was no chapter on ecological development because there was no coherent theory linking these phenomena together, and I couldn’t write a chapter of mere episodes. Cor told me to find such a link. And that year (1995), three publications came out. Firstly, Lynn Nyhart’s book ‘Biology Takes Form’, showed me that the earliest experimental developmental biology was indeed eco-devo. Then, Jessica Bolker had a wonderful article on how the model organisms that we use in developmental biology were all chosen to reduce or eliminate environmental effects, so you could compare your results to the results between labs and highlight genetic causation. And then David Epel wrote that insightful paper, ‘Beakers versus breakers: how fertilisation in the laboratory differs from fertilisation in nature’, discussing how studying sea urchin fertilisation is in the laboratory was different from studying it in the field. At this point I thought, ok, I can now write a coherent chapter on the environment and development that would fit into the textbook.
To play devil’s advocate on your comment about information being so readily available on the internet: why is your textbook still important?
Textbooks summarise, organise, and synthesise an enormous amount of research. They also are de facto ‘gateways’ that interpret what is considered accurate scientific fact. This last is a problematic function because it can prevent new ideas from becoming accepted. However, I think that it’s more important than ever to have someone or some group vetting scientific information.There is so much garbage and misinformation on the web, especially if one is looking for information about fertilisation or human development. Initially, I hated the idea of textbooks being gatekeepers; but that’s what they’re becoming.I think textbooks are becoming an important resource inthe age of the internet and social media, where everybody can be a broadcasting company.In 1994, we started a website for the textbook so that researchers could update, disagree with, and download new information onto the website. We didn’t want the book to be a gatekeeper; we wanted it to be a community resource. We tried that for four years, but it didn’t work, people were not downloading anything. (We had a Quadra 950 computer that could hold 16 people at a time, and a LaCie storage disc that could hold 1 entire gigabyte of information! Yes, it crashed.) But I think, as the internet has become a huge part of our lives, the idea of vetting information has become critically important in science. So, I think that in a crazy way, textbooks have increased in importance.
What’s new in the upcoming (13th) edition of ‘Developmental Biology’?
We have two new and important chapters. One new addition comes straight after the chapter on fertilisation and gives an overview of early development throughout the animal and plant kingdoms. So, before going into the details of sea urchin, Drosophila, chicken, mammalian development, we provide the reader an overview of cleavage and gastrulation, and how it’s done. We talk about the mechanics of cell division, convergent extension, all these things that are going on in different ways in the different phyla. The idea is that we show the forest before we show the trees. The second new chapter is on human embryology. The inclusion of this chapter allows us to discuss things that we couldn’t detail in the mammalian development section. For instance, we can now detail the research on implantation and discuss the fascinating studies on how the embryo and the uterus interact during development. It also meant that we could bring forth some of the bioethics issues. For instance, we talk about when human life begins and also about what is ‘normal.’ (We found eight definitions of what is normal in the biomedical literature.) This chapter will be provocative. It should make people question their ideas. It may not change them, but I think it will give students something to think about and something to talk to their roommates and families about.
How has writing the book changed since Michael Barresi came onboard as your co-author?
The book has benefitted enormously from Michael’s passions and expertise. He is uniquely qualified, as he is pleasantly obsessed by those areas of developmental biology that are growing in importance – developmental neurobiology, environmental disruptions, and stem cells. He has been adamant that plants be fully represented, and that human embryology be a separate chapter. Michael is also doing pioneering work in electronic pedagogy and has helped keep developmental biology teachers active during the Covid-19 pandemic. He is using such electronic pedagogy to show students that developmental biology is an international, interracial, intergender discipline, and that anyone in his class should feel comfortable going into it. He has become the point person for the e-book and for the web-based material. Michael is also teaching classes (whereas I am retired), so he gets immediate feedback from his students, which is invaluable.
What do you think are the next big questions for developmental biologists?
I obviously think that ecological developmental biology is going to be enormously important because it addresses some of the problems of climate change. Climate change is happening, and developmental biology belongs in the context of climate change: what is going to happen to turtles (who have temperature-dependent sex determination), what is going to happen to coral reefs (where the life of the coral depends on its endosymbiotic algae)? What happens to the interactions between plants and their pollinators if a flower’s sexual development is determined by photoperiod, and the pollinators’ eclosion depends on temperature? These all depend on development.
Related to that, I think that studying plant and fungal development is becoming even more important. Our foods, our building materials, perhaps even our energy sources, may be coming from modified plants and fungi. Knowledge of plant and fungal development may be critical for all life on this planet.
Thirdly, I think that brain development will always be one of the great frontiers of developmental biology. As Young Frankenstein says, “Hearts and lungs are simply tinker toys when stacked against the brain!” Liver cells have may have a dozen or so connections to other cells, while brain neurons can have 105 such connections. How are these organized?
Then of course, there is evo-devo. I think that if we are going to discuss the origins of biodiversity seriously, then we have to discuss evo-devo. Nothing about evolution makes sense except in the light of developmental biology. If one wants to talk about how variations occur, you need developmental biology. I’ve started to think about evo-devo in terms of it being the holobiont that is evolving and developing. Then the question becomes: What happens when an organism acquires a new symbiont, does that give it a different phenotype? (We know that some of our agricultural pests used to be rather benign critters until they found a different symbiont, for example the red turpentine beetle, it wasn’t a pest until it found a different fungus.) Our genome has and uses several genes from retroviruses, and some beetles seem to have acquired genes for cellulose digestion from symbiotic fungi. Acquiring symbionts was critical for the evolution of herbivory, and the bacteria helps the bovine rumen to develop! So, looking at the evolution of the holobiont in an evo-devo way is going to be really interesting, and very important.
Finally, is there anything that the Node community might be surprised to find out about you?
I have played piano in a klezmer band and have given concerts of “Schlock Rock of the 60s.” My teaching philosophy comes largely from what Leonard Bernstein said when he was asked why he conducted so much of Mahler’s music. Bernstein replied that since he loves Mahler and loves his audience, he wanted his audience to love Mahler as much as he did. It’s easy to transfer that to developmental biology.
Thank you for taking the time to interview me.


 (5 votes)
(5 votes)