Minipigs get stem cell transplants to treat blindness
Posted by Joyce Yu, on 20 December 2024
PRESS RELEASE: Millions of people around the world are affected by retinal degenerative diseases. In most cases, loss of vision is caused by damage to the macula, a region in the centre of the retina. The macula is rich in cone photoreceptors – cells important for perceiving colour and seeing finer details. Currently, there are no approved treatments to replace the damaged macula, despite its huge impact on the quality of life. Now, a team of researchers from the University of Montreal, led by Professor Gilbert Bernier, found that blind minipigs receiving retinal transplants made from stem cells showed signs of restored vision. They published their study in the journal Development on 5 December 2024.
In this study, Professor Bernier’s team developed a method to coax stem cells into forming sheets of cells that recapitulate the structure of the human retina. The type of stem cells they used are called human induced pluripotent stem cells – immature cells ‘reprogrammed’ from an adult (mature) cell that can differentiate into any type of cells in the body. Using the stem cells, the researchers made ‘retinal sheets’ that are enriched in immature versions of the cone photoreceptor cells, which could become mature cone cells when cultured in the lab.
After successfully creating the retinal sheets in a dish, the researchers tackled the next challenge: transplanting these sheets into minipigs with damaged macula. Professor Bernier explains, “To get as close as possible to human clinical application, we have chosen minipigs because the size of their eyes is near that of humans and the animals are about the same weight as humans. Hence, all surgeries in our study could be performed by a retinal surgeon.”
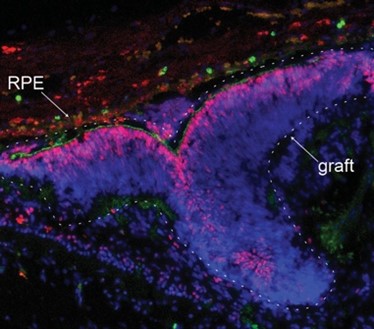
Upon transplantation, the researchers found that the retinal grafts were able to integrate into the minipig’s damaged retinal tissue. Encouragingly, the minipigs showed signs of restored vision: new neural connections were formed between the grafted photoreceptor cells and the minipigs’ neural cells, and the scientists could detect neural activity of the photoreceptors at the grafted area when the minipigs were placed in a well-lit room.
Given the pressing need to develop therapeutic interventions against vision loss, researchers around the world are testing different ways to repair damaged macula. “Some approaches use dissociated photoreceptor cells; others create micro-dissected retinal organoids, which are lab-grown ‘mini-organs’ in a dish,” says Professor Bernier. “In contrast, our method allows the spontaneous formation of a flat retinal tissue that is already polarised and organised, as in the human embryonic retina.” He adds that their method can generate large yields of retinal tissue for transplantation.
A limitation in this method lies in the difficulty of controlling the placement and orientation of the grafts during surgery. The macula is only 4mm in diameter – about the length of a grain of rice. “To properly orient, place and stabilise the graft in the retina remains a big surgical challenge,” says Professor Bernier. His team are now working to improve the transplantation success rate. They are validating an experimental retinal surgery device to ensure proper orientation and implantation of the graft at the correct retinal disease site. Although many challenges remain, this study demonstrates the potential of retinal sheet transplantation for treating retinal degenerative diseases.
REFERENCE:
Barabino, A., Mellal, K., Hamam, R., Polosa, A., Griffith, M., Bouchard, J-F., Kalevar, A., Hanna, R., Bernier, G. (2024) Molecular characterization and sub-retinal transplantation of hypoimmunogenic human retinal sheets in a minipig model of severe photoreceptor degeneration. Development, 151, dev203071. doi:10.1242/dev.203071


 (No Ratings Yet)
(No Ratings Yet)