YEN Image Competition 2025
Posted by Matyas Bubna-Litic, on 20 May 2025
The winners of the Young Embryologist Network (YEN) 2025 Image Competition have been announced at the conference today held at the Francis Crick Institute.
Please check out the top 10 images that have received the most votes from attendees of the YEN 2025 Conference.
We thank those who sent us their images and attendees of YEN 2025 for helping us select these images. If you would like to learn more about YEN visit www.youngembryologists.org
YEN Image Competition winner:
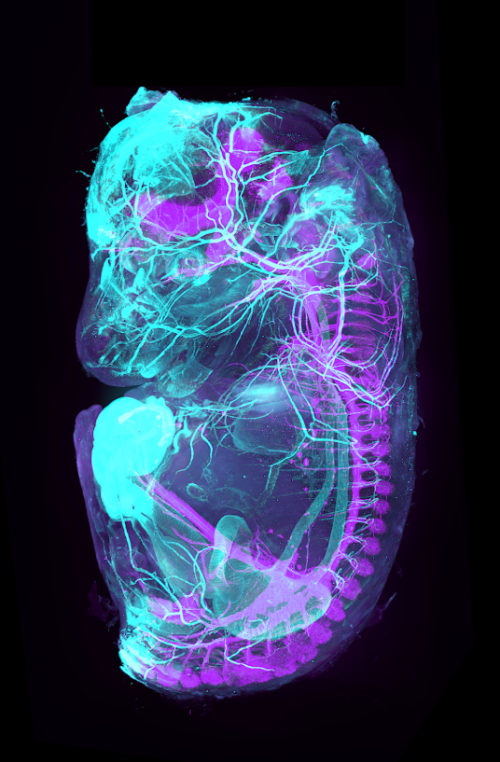
Théo Morel (PARCC Paris, Inserm-UMR970, France)
A 3D view beyond the skin
What if we could directly visualize the arterial and nervous systems behind the skin of a mouse embryo? To make this possible we used a iDISCO clearing protocol on a 15 dpc mouse embryo, followed by whole mount co-staining with ASMA (light blue, marking arteries) and TH (purple, marking the sympathetic nervous system). The embryos were imaged using light-sheet microscopy and reconstructed in 3D using Imaris software.
Runner-up:
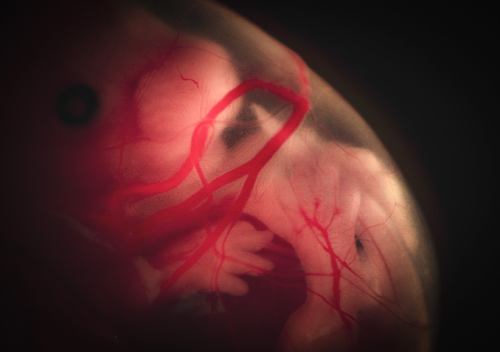
Lisa Leinhos (University of Oxford, UK)
Cosmic life
Drifting in the womb like an astronaut in space, the embryo floats peacefully, a moment of cosmic calm in the universe of early life. This image portrays a dissected mouse embryo at E14.5, captured through a Nikon camera on a binocular microscope system. The focus of this experiment is to investigate gene expression at different stages of embryonic development using bulk RNA sequencing. What makes this image particularly compelling is the contrast between its scientific significance and emotional depth. Although the embryo is no longer alive, the image portrays a moment of serene stillness, almost as if it were still within the womb. It captures the profound beauty of life’s early stages while highlighting the mysteries that science seeks to understand. At the same time, there is an inherent sadness, as this specimen, like many others, has been sacrificed in the name of advancing our knowledge of developmental processes. This delicate balance of scientific discovery and respect for life makes the image not only visually striking but also emotionally resonant.
Runner-up:
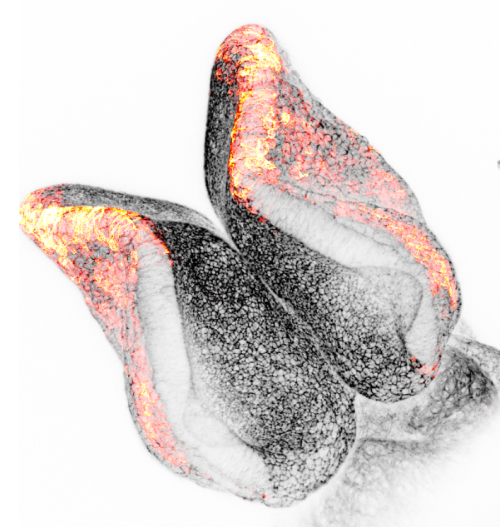
Andrea Krstevski (Institute of Child Health, UCL, UK)
Minnie’s Bow
Neural crest cells play a crucial role in neural tube development as they are a population of multipotent cells that emerge from the dorsal neural tube. These cells migrate to various regions of the embryo and differentiate into a diverse array of tissues, including neurons, glial cells, and components of the peripheral nervous system. Their proper development and migration are essential for the correct patterning and function of the nervous system. Any disruption in neural crest cell development can lead to a variety of congenital disorders and malformations. The image shown is a E8.5 mouse embryo displaying actin in black and migrating neural crest cells using marker Pax3 in red. The Zeiss LSM 880 upright confocal multiphoton microscope was used to capture the image.
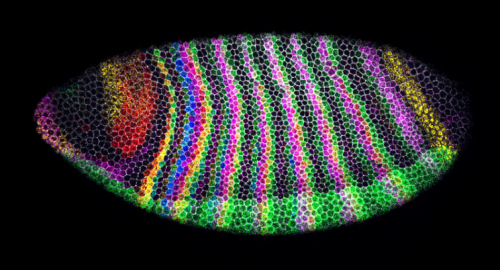
Swanee Douglas and Dr Tom Pettini (Department of Genetics, University of Cambridge, UK)
Segment-polarity stripes
Drosophila embryo with immunostaining of cell membranes (grey) and 5-plex HCR in situ hybridization to visualize segmentation genes (blue = engrailed mRNA, green = paired + snail mRNA, magenta = odd-skipped mRNA, yellow = wingless mRNA, red = sloppy-paired 1 mRNA) with minimal crosstalk, Microscope: 40X Z-stack on Stellaris 5 confocal microscope (sum projection here), Processing: Python and Fiji

David Grainger (Institute of Developmental and Regenerative Medicine, University of Oxford, UK)
SMILE
This image showcases a vibratome section of an E9.5 mouse embryo, where KDR immunostaining (magenta) delineates endothelial progenitors and TUBB3 (green) marks developing neurons. Captured using a Zeiss LSM980 confocal microscope, a maximum intensity projection and horizontal mirroring were applied to optimize signal and symmetry.
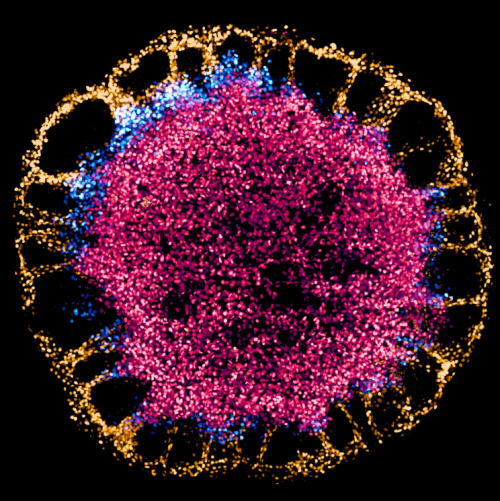
Francesca Montesi (The Francis Crick Institute, UK)
A blossom of stem cells
Human embryonic stem cells differentiating on a 1000 µm-diameter hydrogel micropattern. Cells differentiate and form hollow cysts at the periphery, while they remain pluripotent at the core of the colony (CDX2, gold; BRA, blue; SOX2, red). Imaged on a spinning disk confocal microscope and processed with Imaris.
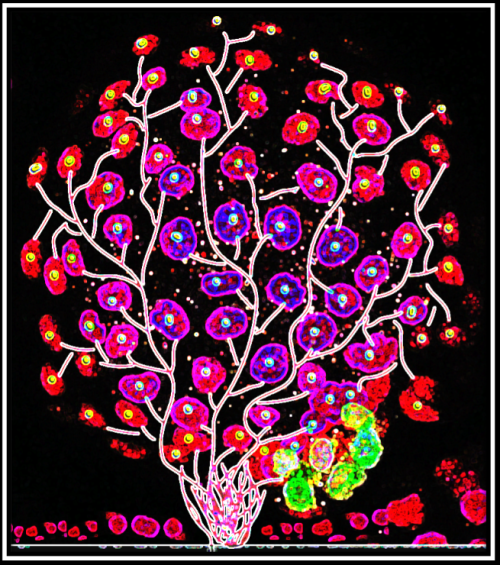
Jinlong Qiu (Hull York Medical School)
Blossoming Embryo
A fluorescence microscopy representation of a bovine blastocyst, stained with Hoechst 33342, Alexa Fluor 488 conjugated with NANOG and Alexa Fluor 568 conjugated with GATA6. The image was captured using a Zeiss LSM710 confocal microscope and later artistically modified. Tree-like branches were digitally illustrated using Adobe Illustrator, and further contrast adjustments and artistic enhancements, including the ‘Glow Edges’ effect, were applied in PowerPoint. This fusion of science and art transforms the cellular organization of early embryonic development into a vibrant visualization of growth and differentiation, resembling a flourishing tree of life.
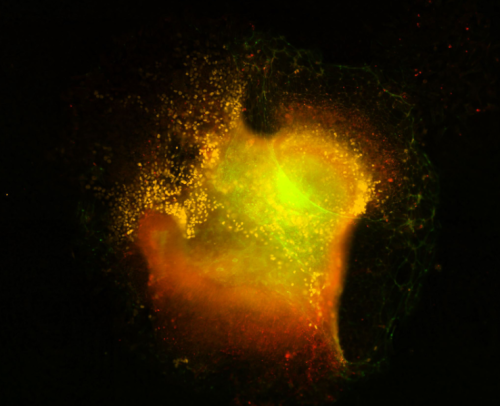
Achira Karunaratna (Institute of Child Health, UCL, UK)
Crest Nebula
Cranial neural crest explant visualized via immunofluorescence after staining for marker proteins. The image was captured on the Nikon eclipse Ti2 series epifluorescence microscope housed at UCL GOS ICH at 20x followed by gaussian stack focusing of a Z series. Explanting is a widely used ex-vivo approach to study neural crest development in animal models. The neural crest, regarded as the ” fourth germ layer”, gives rise to multiple important cell types within vertebrate bodies, including the craniofacial cartilage, peripheral nerves, and pigment cells among others. This explant experiment is part of a wider attempt to understand the mechanisms of neural crest migration that contributes to neural tube defects in mammalian embryos by understanding cytoplasmic protein methylation in migrating g neural crest cells. Visualised in yellow are neural crest cells (stained for neural crest marker sox10 ) emerging out of the mouse cranial neural folds. Tinges of green at the outer edges represent phalloidin staining marking F-actin localization in cells. The faint red hue marks another membrane-bound protein SETD2, a methyltransferase, within the explant and in migrating cells. The circular looping where neural crest cells emerge is reminiscent of areas of clouds of gas and dust that form when stars are born in nebulae in the pitch-dark corners of our universe. Hence, aptly named a crest nebula, a factory for neural crest cell production.
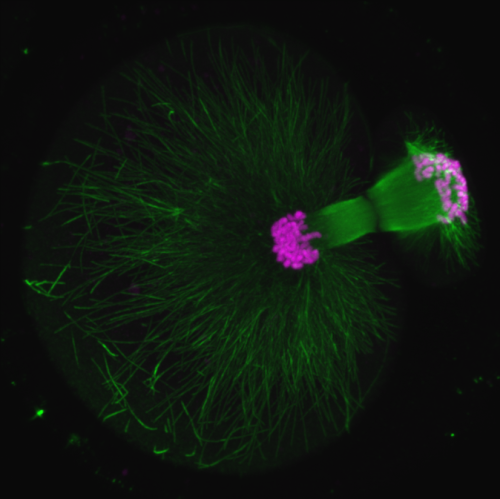
Ninadini Sharma (Biology & Biological Engineering Caltech, US)
Polar body extrusion
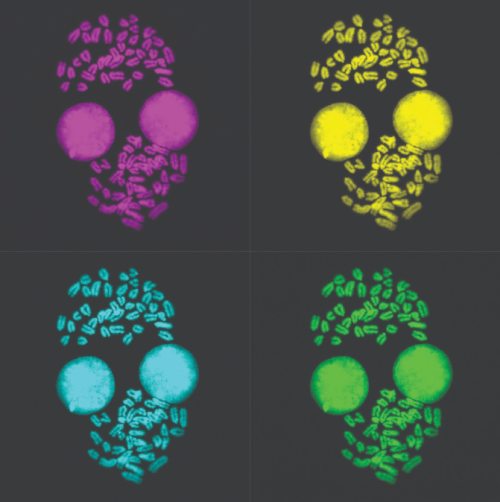
Malgorzata Borkowska (MRC Laboratory of Medical Sciences, Imperial College London, UK)
Metaphase Monroe
Metaphase spread of 2i/LIF grown mouse embryonic stem cells image obtained with Leica SP5 confocal and processed in Fiji for chromosome counting.


 (No Ratings Yet)
(No Ratings Yet)