A day in the life of a Kabuto-mushi (rhinoceros beetle) lab
Posted by Shinichi, on 10 December 2018
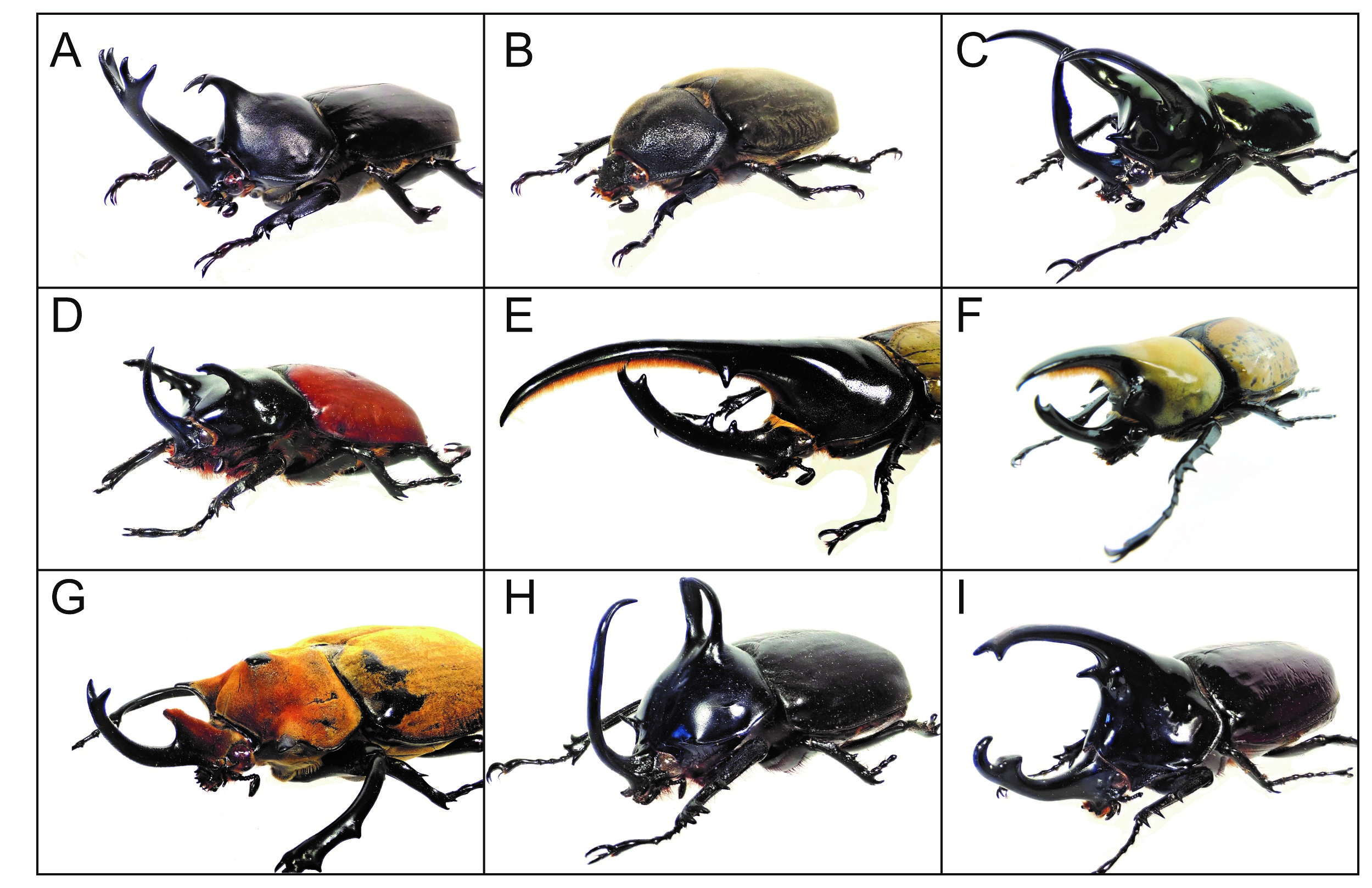
I am Shinichi Morita, a postdoctoral researcher in Teruyuki Niimi’s lab at the National Institute for Basic Biology, Japan (Fig. 1A, B). Our research interests focus on the evolutionary novelties that insects have acquired, and how various insect morphologies have arisen during evolution (Fig. 1C-P).

Beetle horns are thought to be an evolutionary novelty and are used as weapons for intraspecific combats between males. Beetle horns display sexual dimorphism in many Scarab beetles, and their shapes, numbers, sizes and forming regions are highly diverged even among closely related species (Fig. 2). Elucidating how these novel traits were acquired in Scarab species will lead to better understanding of the mechanisms of morphological diversification during evolution. My postdoc project is to understand how exaggerated horns are acquired and formed in Japanese rhinoceros beetles (Trypoxylus dichotomus) (Fig. 2A, B).
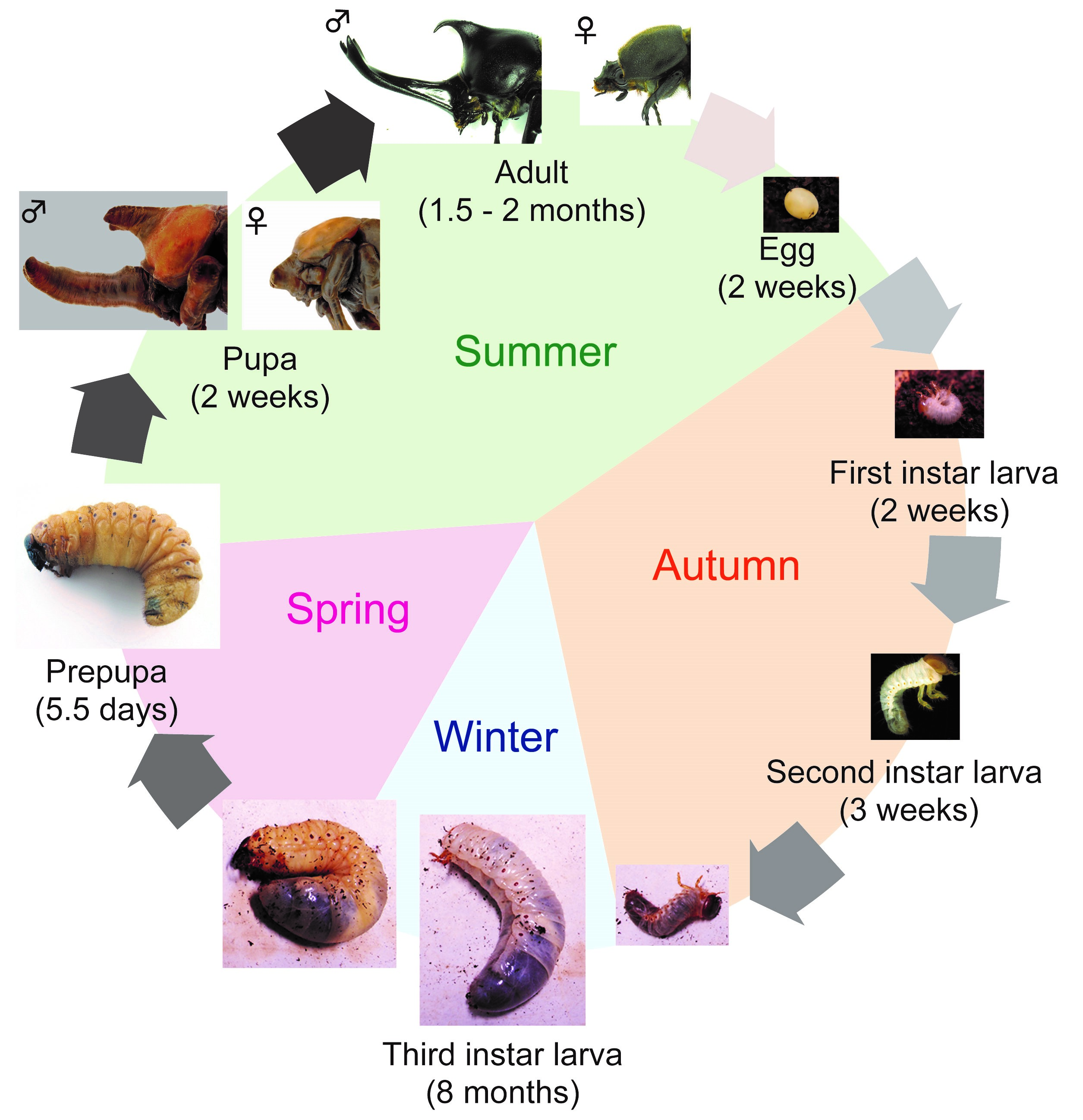
First, I will introduce T. dichotomus. In Japan, T. dichotomus is a popular and familiar insect to children and adults, and is sold as a pet at department stores and DIY shops during the summer. The Japanese name for T. dichotomus is Kabuto-mushi, and it is also used as one of the season words of “Haiku” (Haiku is a traditional short Japanese poem with seventeen syllables in the pattern of 5-7-5, including a season word in it). T. dichotomus is a holometabolous insect that has egg, larval, pupa and adult stages. Its life cycle is about 12 months (Fig. 3). Adults emerge in early summer and lay eggs in the soil at the end of summer. Larvae hatch from the eggs in about 2 weeks (Fig. 3, Egg) and feed on humus. The larval period is about 8 months and they pupate in the next spring (Fig. 3, First – Third instar larva). At the end of third (last) instar, they make pupal chamber (Fig. 3, Prepupa). During the prepupal period, horn primordia are formed in the head and the thorax. After about 2 weeks of pupal period (Fig. 3, Pupa), they become adults in the early summer (Fig. 3, Adult). T. dichotomus male adults have exaggerated horns on the head and prothorax (Fig. 2A), whereas females do not have these structures (Fig. 2B). The head horn is shaped like a plow with a long stalk, and bifurcated twice at the distal tip, while the prothoracic horn is shorter than the head horn, and bifurcated once at the distal tip.
Here, I share my daily activities in the laboratory. As mentioned above, since the life cycle of T. dichotomus is one year, our daily life differs depending on season. In April, we purchase about 3,000 – 4000 last instar larvae of T. dichotomus from insect supplier (Fig. 4A). With help of our lab members, the sex of these larvae are determined (Fig. 4B) and they are packed individually in a bottle filled with breeding mats (Fig. 4C). They are stored at 10 ° C until use (Fig. 4D). Therefore, we can use T. dichotomus as an experimental material in any season.
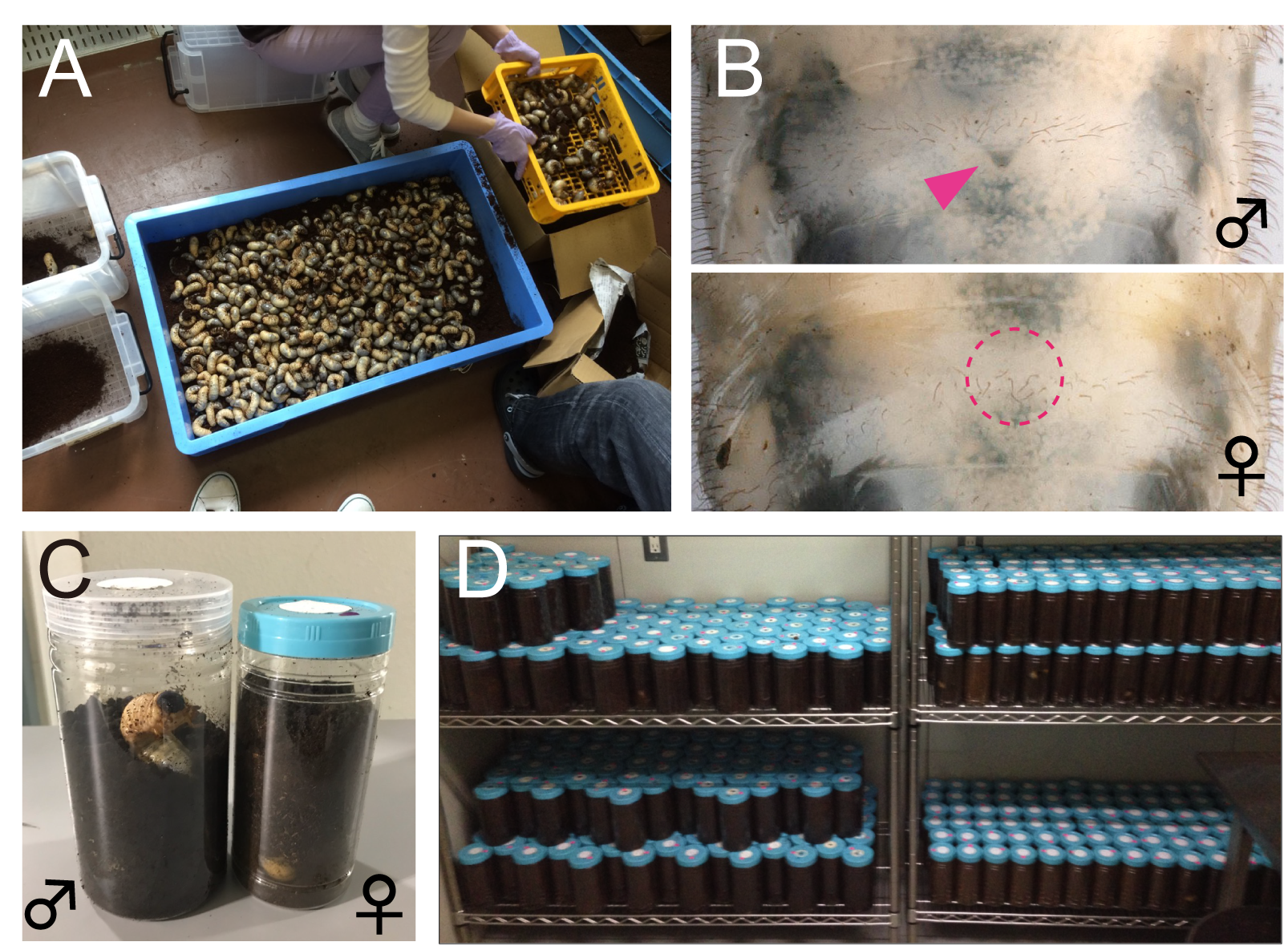
(A) Purchesed last instar larvae of T. dichotomus. (B) The male and female larvae are discriminated by the presence (male) or absence (female) of the Herold’s organ. (C) The larvae are reared individually in a container (140 mm in height and 95-mm in diameter for males, 130 mm in height and 75 mm in diameter for females) filled with breeding mats. (D) Larvae are stored in a low-temperature room.
The sexual dimorphism of horns first appears in the horn primordia during the prepupal stage (Fig.3, Prepupa). One of my main research aims is to find out which genes control horn formation, so we dissected horn primordia in males and females at this stage and performed comparative transcriptome analysis by RNA-seq. We performed an intersexual comparison between male and female horn primordial transcriptome datasets, and tried to identify genes driving the development of different morphologies between male and female. In addition, we also compared transcriptome data between different horn types (head and prothoracic horns) intersexually in males and females. As a result, we identified 1,553 differentially expressed genes (DEGs) in total. To identify the essential genes for horn formation, we focused on genes encoding 38 transcription factors and 11 signaling molecules included in the 1,553 DEGs, and performed larval RNA interference (RNAi) screening. In beetles including T. dichotomus, larval RNAi experiments is extremely efficient to analyze the function of genes during postembryonic development. Double-stranded RNA (10 – 50 µg) was injected laterally into the T1 segment of each last instar larva before the prepupal stage using a 1 ml syringe with a 30 gauge needle. As a result, 11 genes were newly identified as horn formation genes (Fig. 5A-E). Interestingly, these 11 genes are mostly categorized as larval head- and appendage-patterning genes.
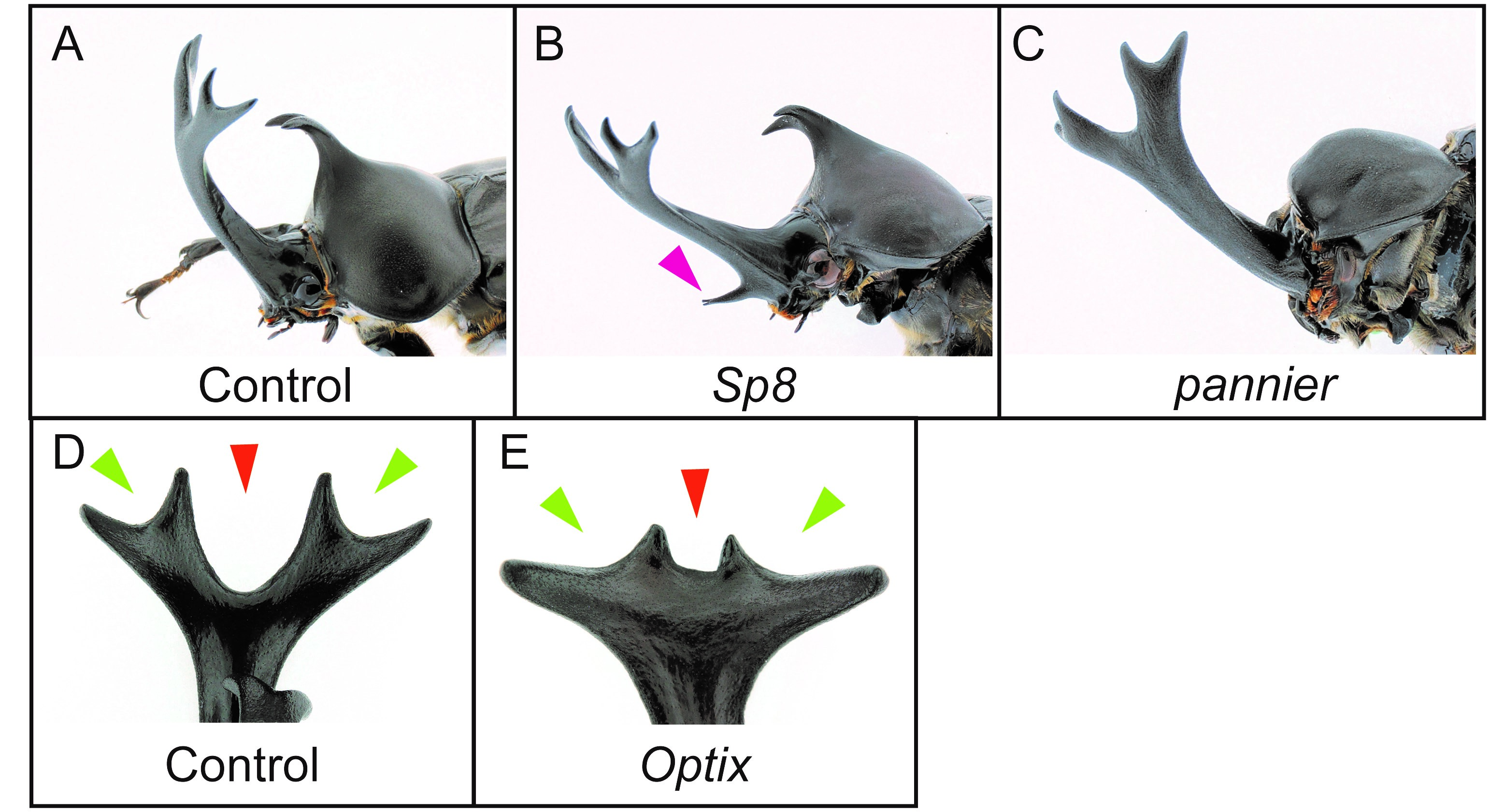
(A) Control RNAi phenotype (EGFP). (B) Sp8 RNAi phenotype. (C) pannier RNAi phenotype. (D) Dorsal view of a head horn tip in control RNAi phenotype (EGFP). (E) Dorsal view of a head horn in Optix RNAi.
Future perspective of beetle horn studies
In addition to the above 11 genes, we have identified a number of other horn formation genes, but the gene regulatory network for horn formation is still unknown. So far, we have set up various resources and tools necessary for beetle horn research (precise staging of beetle horn formation, larval RNAi, whole mount in situ hybridization, immunostaining etc.). Therefore, it has become possible to analyze the horn formation gene regulatory network through developmental biological approaches. In addition, we are going to test whether novel cis-regulatory elements play a crucial role in acquisitions of horns during evolution. To this end, we are planning to perform ATAC-seq (Assay for Transposase-Accessible Chromatin sequencing), a technique to assess genome-wide chromatin accessibility, as a project to estimate cis-regulatory elements involved in horn formation and acquisition. Through the above approaches, we believe a part of evolutionary mechanisms associated with acquisition of animal diversity will be unravelled at the molecular level.
If you would like any more information about rhinoceros beetles and evo-devo questions in Scarab beetles, please get in touch with me (shinichi@nibb.ac.jp) or Teruyuki Niimi (niimi@nibb.ac.jp).
References
- Ohde, T., Morita, S., Shigenobu, S., Morita, J., Mizutani, T., Gotoh, H., Zinna, RA., Nakata, M., Ito, Y., Wada, K., Kitano, Y., Yuzaki, K., Toga, K., Mase, M., Kadota, K., Rushe, J., Lavine, LC., Emlen, DJ. and Niimi, T. (2018) Rhinoceros beetle horn development reveals deep parallels with dung beetles. PLOS Genetics, 14: e1007651.
- Ito, Y., Harigai, A., Nakata, M., Hosoya, T., Araya, K., Oba, Y., Ito, A., Ohde, T., Yaginuma, T. and Niimi, T. (2013) The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle. EMBO reports, 14: 561-567.


 (8 votes)
(8 votes)
please see http://www.embryogeometry.com for a rhino beetle picture that will interest you
Just a beetle lover, and this is so interesting! The Figures are so nicely pictured and drawn. I hope everything is going well in their labs