A day in the life of a siphonophore lab
Posted by cmunro, on 27 October 2015
I’m Cat Munro, a third year PhD Candidate in Casey Dunn’s lab at Brown University. The Dunn lab has an even split of lab members that work on the evolution, development, and systematics of siphonophores, and members that focus on building tools and phylogenetic methods, with an eye to understanding the relationships at the base of the animal tree.
So what are siphonophores, and why would anyone study them?
Siphonophores
Siphonophores are cnidarians, more specifically, they are found within the clade Hydrozoa. Like many other hydrozoans they are colonial, reproducing asexually to produce a colony of clonal, physiologically integrated, and physically attached bodies. However they have such a high degree of physiological integration that we consider each of these ‘bodies’ (termed zooids) to be homologous to a solitary, free living individual (think of a solitary polyp, like Hydra or Nematostella). Each of these zooids is also functionally specialized, and show a division of labour – some zooids are specialized for locomotion, others for feeding, reproducing, digesting, protecting and so on. As a lab, we study siphonophores in order to understand the evolution of this type of functional specialization.
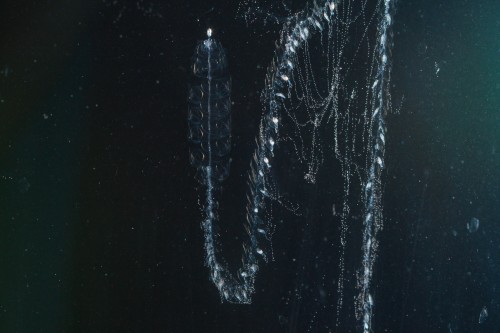
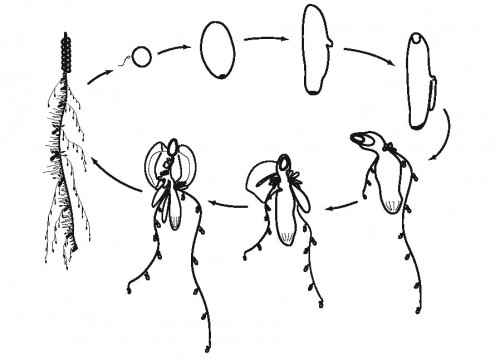
There is a sexual phase in the life cycle, where fertilized eggs develop into a primary feeding zooid with a gas-filled float, that subsequently forms two growth zones (in some species there is only one). The growth zones are the site of asexual budding. In most species the zooids are produced from a single probud, that subdivides further to form various zooid types along the stem. The zooid types are always produced in the same reiterated sequence. What’s great about this form of development is that we have access to an entire ontogenetic sequence along the stem of the colony, with the oldest zooid at one end and the youngest probud at the other end in the growth zone. Additionally, because the organization is highly organised and predictable, we always know the zooid identity of each bud in the growth zone.
A typical (field) day
A typical day in a siphonophore lab might include time at the bench working up samples for sequencing or time at the computer working on data analysis. We also spend time on the microscope working with fixed and stained samples. However, these late Summer/Fall field days provide us with hundreds of samples that last for months until we get back out to the field in the spring and summer.
0430: The alarm clock rings. After a few minutes feeling disorientated by the early wake up, I remember – it’s dive day. I get up, throw on my swimsuit and clothes, and make a quick breakfast.
0500: Just before heading out the door, I check the marine weather forecast. We check the weather a lot in the days before a field day. If the wind is blowing faster than ~25 knots and wave height is higher than 5 ft/1.5 m, then it becomes harder (and even dangerous) to get in the water and collect specimens. The seas were rough the past few days, but the weather looks good, so I jump in my car.
0630: I arrive at Point View Marina, South Kingston, Rhode Island. The sun is rising, and a few fishermen are already starting up the engines of their boats, coffee in hand, to start a day of fishing. The dock poles here are adorned with billfish bills and tails from other successful fishing expeditions.
Along with the rest of the lab, we empty the truck and load up the boat with our equipment (coolers with plastic collecting jars; buckets and a plankton net; dive gear and tanks).

0700: The Captain gets the engine going and we leave the dock. The boat slowly makes its way out into Point Judith Pond, a well sheltered body of water that opens out into Block Island Sound. To our right, we pass sleepy vacation homes, some still occupied by a few holdouts at the end of summer; and to our left, a large fishing vessel is docked in the port of Galilee, where among a flurry of seabirds, fishermen are already unloading the day’s catch.
As we leave the relative calm of Point Judith Pond, the waves are higher and the boat speeds up. We settle into our seats, we have a few hours until we reach our destination.
~0900: The engine cuts. The captain has been waiting for the waters to get bluer, and perhaps also some indication of a scattering layer in the sonar. We check the water to see if we can see any gelatinous organisms below.
All the divers start to gear up, and in the meantime our other lab members fill the collection jars. The jars are prefilled with seawater, without any bubbles, so that there aren’t any issues with the pressure at depth crushing them or making them hard to open. They are loaded into mesh bags that the divers can clip on to their buoyancy control device (BCD).
0930: We get into the water. The type of diving we do is called blue-water diving which, due to some of the unique issues related to diving in the open ocean with no seafloor in sight, requires special procedures. We need a constant connection to the boat, and so we use a slightly weighted ‘down line’ that connects to a buoy and then to the boat. Each of the divers are connected to their own tethers that are attached to a loop of metal called the trapeze. The trapeze can be clipped onto the down line at any depth, and each of the divers can then spread out to collect our specimens. One diver is the ‘safety diver’ and stays near the trapeze and the down line and keeps an eye on all of the divers.
Blue water diving is unlike any other diving experience. Without the seafloor as a frame of reference, it can be quite disorientating, and it is possible to shift depths quite rapidly (this is part of the reason for the tether system). On a sunny day, when you look down, you can see shafts of sunlight that extend down and gradually fade into a dark blue. Sometimes there are Sapphirina copepods below us that flicker back blue light from their skin as they move; against the background of the darker deep water they look like shimmering stars. It’s beautiful, but unless you’re the safety diver, you don’t have much time to take it in. We’re on a mission to spot and catch siphonophores, and today it isn’t hard – they’re all around us, alongside several species of ctenophore, various hydromedusae, and pteropods. It’s a gelatinous soup.
Catching siphonophores can be hard. They are largely transparent, and in very clear waters you often need to angle your head to catch the right angle of light, or use the dark suits of your fellow divers for contrast. We catch our specimens by opening the lids of the jars and gently swirling the animal into the jar. Sometimes before you collect, you have to poke them gently so that they retract all their tentacles.

1030: The dive is over. We’ve gradually made our way up the down line from 60ft, and once our air reaches 500 PSI, we take a safety stop and ascend. Usually the limit is not air, but the number of jars, and by the end of the dive we are checking our samples and evaluating whether it is worth abandoning one sample and collecting a particular species. Back on the boat, we check our haul. Based on numbers, we reassess which species we should collect on the next dive. The jars are loaded into the coolers with some ice packs to keep them cool, and more jars are filled with seawater for the next dive.
1100: We get back in the water and descend to 60ft. Sometimes we find that even though we are roughly in the same location, the patch of water can change slightly, and we see slightly different species assemblages to the last dive. Sometimes even within a single dive, if there is a reasonable current, we can see very different species throughout the dive.
1200: We finish up the dive and get back on the boat. We load up the last of our samples, grab a few carboys of water from the site, quickly change out of our wetsuits, and pull the lines out of the water. Within 30 minutes or so, the captain fires up the engine and we head back towards the marina. Generally we are exhausted after the dives, so we eat our packed lunches and fall into a deep sleep until the boat pulls back into the dock.
1500: The boat is back in the dock and it’s time to unload. Everyone has just woken up from their naps, so we stop for a quick coffee on our way back to lab.

1600: Back in the lab, we do our final counts of different species and transfer our samples into the incubator. We need to work out the fate of each of the samples for various projects: some of the samples are frozen for phylogenetics, differential gene expression studies, and also fixed and stored for in situs and immunostaining. Most of the samples need to be processed the next day, but others need to be processed right away.
Along with a fellow grad student, I spend a few more hours in the lab processing samples for various projects.
2200: Finally home! Once I rinse off all of my dive equipment, I can finally shower and clamber into bed. I’ll sleep well, but not for too long – there are more samples waiting to be processed in the morning.
 This post is part of a series on a day in the life of developmental biology labs working on different model organisms. You can read the introduction to the series here and read other posts in this series here.
This post is part of a series on a day in the life of developmental biology labs working on different model organisms. You can read the introduction to the series here and read other posts in this series here.


 (10 votes)
(10 votes)
Super nice.
It’s so cool to read this session of the blog and find out that there are many labs working on non-classical models. I’ve read your paper on Nanomia and I find them very inspiring. I hope I get the chance to write a day in the life of a sponge lab!
I’m so jealous of your siphonophore collecting day. Thanks so much for this wonderful post, beautifully written!