A TALE OF LIMBS AND GENITALS
Posted by Patrick Tschopp, on 17 November 2014
The morphological evolution of limbs and external genitalia were both essential adaptions to a life on land. While the former deals with the novel locomotory challenges facing an animal invading a terrestrial environment, the latter is concerned with something even more essential: reproduction! Living on land means that gametes can no longer be fertilized externally simply by releasing them in water, e.g. as frogs do. Male and female gametes need to be brought together as well as protected from dehydration. Internal fertilization, utilizing specialized external genitalia, solves this dilemma, delivering sperm to their target and hence providing protection inside the animal’s body.
In addition to the adaptive value of limbs and external genitalia in the context of the transition to land, there is also a striking similarity in the patterning genes expressed during the development of these two structures. For both these reasons, a potential co-evolution of limbs and genitals has been discussed in the past1-4. In our recent Nature paper we have identified another, unexpected link between the limbs and external genitalia that may help to explain some of the molecular similarities between the two5.
From Limbs to Genitals
As might be expected for Cliff Tabin’s lab, this project also started out as a study focusing on limb development. At the time, Jérôme Gros (a former postdoc in the lab, now PI at Institut Pasteur, Paris) was investigating the earliest steps of limb initiation in vertebrate embryos. He found that in both mouse and chicken embryos the mesenchyme of the growing limb buds originates from the epithelial lateral plate mesoderm (LPM), through a process called epithelial-to-mesenchymal transition, or EMT6. Given the interest of our lab in evolutionary questions, former graduate student Jimmy Hu suggested looking for the presence or absence of the same process in the limb-less snake….of which he just happened to have a few embryos in his freezer – courtesy of Olivier Pourquié who had previously brought to our attention the presence of outgrowths at a hindlimb-like position. There was no evidence for such EMT at the level where forelimbs once were supposed to form. However, when looking at the hindlimb level, a clear EMT was visible – only in snakes the resulting mesenchymal cells of this “limb-like” bud contributed to their budding external genitalia, the so-called hemipenes (Figure 1). Such similarity in limb and genital bud initiation in squamates led us to question the developmental origin of external genitalia in other amniotes.
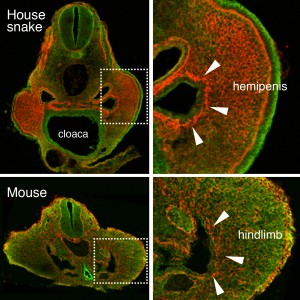 Figure 1. An epithelial-to-mesenchymal transition (EMT) underlies the developmental initiation of the house snake hemipenis and the mouse hindlimb bud. Breakdown of the basement membrane (laminin staining in red) is seen in both embryos (arrowheads).
Figure 1. An epithelial-to-mesenchymal transition (EMT) underlies the developmental initiation of the house snake hemipenis and the mouse hindlimb bud. Breakdown of the basement membrane (laminin staining in red) is seen in both embryos (arrowheads).
Tracing the Origin of External Genitalia
Squamates (snakes and lizards) are interesting in this regard, as within their clade partial or complete loss of limbs occurred among several species, yet they all keep their hemipenes. Using micro-computed tomography in collaboration with Emma Sherratt (now at University of New England, Australia), we realized that all squamate embryos formed their hemipenis buds at the same level where hindlimbs would form, whereas the mouse genitalia were emerging more posteriorly, towards the tail (see video). Moreover, we were able to visualize the internal location of the cloaca, an endodermal signaling center known to be important for genital outgrowth. Intriguingly, the cloaca seems similarly shifted in all squamate embryos, into the presumptive hindlimb field. This prompted us to determine which cell populations actually give rise to the different species’ genitalia – a question that seemed far from settled, when consulting the available literature.
Using a lentiviral lineage tracing system we were able to demonstrate that, indeed, important differences exist in the developmental origin of external genitalia among different species: whereas the mouse genital tubercle is built mostly of tailbud-descendant cells, the Anolis lizard hemipenis consists of cells from the same embryonic lineage that gives rise to its hindlimbs. In both species the external genitalia thus seemed to “follow” the localized signaling of the cloaca. This suggested the possibility that the evolutionary change in cell populations forming the external genitalia could, at least in part, be attributable to a shift in the relative position of the cloaca.
A Deep Homology of Vertebrate Genitalia
An organ’s transcriptional signature is influenced by its developmental origin, yet can also give hints about evolutionary relations to other tissue types7,8. We therefore performed comparative RNA-seq analyses on early and late budding stages of limbs and genitalia, in both lizard and mouse embryos. Working in distantly related species, while considering similar tissue types, comes with its own set of problems when performing comparative transcriptomic studies – however, after a somewhat rugged start, it soon became clear that the Anolis limb and genitalia transcriptomes show a much higher degree of overall similarity, than was the case for the mouse samples. Also, at early stages, the Anolis hemipenis transcriptome is virtually indistinguishable from a generic limb molecular signature, and only later differentiates into a genitalia-like state. This confirmed, at a molecular level, the relatedness of the cells building limbs and genitalia in the Anolis lizard. Moreover, by grafting the cloacal signaling center into chicken limb buds, we were able to partially induce transcriptional changes reminiscent of early genitalia development, demonstrating the conserved ability of limb cells to respond to these cloacal signals, and supporting the idea that change in the location of the cloaca would have induced a similar genetic program in a different target tissue.
This study offers a potential explanation for the still striking similarities in gene expression in species that develop limbs and genitalia from discrete cell populations4 – namely, that a limb-derived state could represent the ancestral condition for the emergence of external genitalia. As such, a limb-like gene regulatory network for genitalia growth might have become hardwired in a putative ancestral genome. The genitalia of mice and lizards, while not homologous to one another sensu stricto, might thus represent an example of Deep Homology9: with homology in the genetic programs being executed and induced by the same ancestral signaling source, the cloaca.
1. Kondo T, Zákány J, Innis JW, & Duboule D (1997). Of fingers, toes and penises. Nature, 390 (6655) PMID: 9363887
2. Yamada, G., Suzuki, K., Haraguchi, R., Miyagawa, S., Satoh, Y., Kamimura, M., Nakagata, N., Kataoka, H., Kuroiwa, A., & Chen, Y. (2006). Molecular genetic cascades for external genitalia formation: An emerging organogenesis program Developmental Dynamics, 235 (7), 1738-1752 DOI: 10.1002/dvdy.20807
3. Cohn, M. (2011). Development of the external genitalia: Conserved and divergent mechanisms of appendage patterning Developmental Dynamics, 240 (5), 1108-1115 DOI: 10.1002/dvdy.22631
4. Lin, C., Yin, Y., Bell, S., Veith, G., Chen, H., Huh, S., Ornitz, D., & Ma, L. (2013). Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages PLoS Genetics, 9 (1) DOI: 10.1371/journal.pgen.1003231
5. Tschopp, P., Sherratt, E., Sanger, T., Groner, A., Aspiras, A., Hu, J., Pourquié, O., Gros, J., & Tabin, C. (2014). A relative shift in cloacal location repositions external genitalia in amniote evolution Nature DOI: 10.1038/nature13819
6. Gros, J., & Tabin, C. (2014). Vertebrate Limb Bud Formation Is Initiated by Localized Epithelial-to-Mesenchymal Transition Science, 343 (6176), 1253-1256 DOI: 10.1126/science.1248228
7. ARENDT, D. (2005). Genes and homology in nervous system evolution: Comparing gene functions, expression patterns, and cell type molecular fingerprints Theory in Biosciences, 124 (2), 185-197 DOI: 10.1016/j.thbio.2005.08.002
8. Wagner, G. (2007). The developmental genetics of homology Nature Reviews Genetics, 8 (6), 473-479 DOI: 10.1038/nrg2099
9. Shubin, N., Tabin, C., & Carroll, S. (2009). Deep homology and the origins of evolutionary novelty Nature, 457 (7231), 818-823 DOI: 10.1038/nature07891


 (2 votes)
(2 votes)