An odyssey through the genesis of hematopoietic stem cells
Posted by Anne Schmidt, on 21 September 2018
Here, Mylène Lancino and myself will introduce our motivation to investigate and delve deeper into one essential and very peculiar process of Stem Cell Biology: the de novo genesis of hematopoietic stem cells, according to a process referred to as the Endothelial-to-Hematopoietic Transition (the EHT, named initially by Kissa & Herbomel 2010). The work led to a paper published in eLife at the end of last month (https://doi.org/10.7554/eLife.37355). We will explain the technical difficulties inherent to the live imaging and image analysis approaches that have been undertaken. We will also summarize some of our main findings and underline their impact on fundamental and conceptual challenges for the hematopoiesis topic.
In the year 2010, several important papers that unambiguously demonstrated the vascular origin of hematopoietic stem cells were concomitantly published, which shed light on a long standing debate initiated at the beginning of the 20thcentury (Jordan 1917, Sabin 1917; and for a recent review on hematopoiesis reporting on the findings of 2010 by Bertrand et al; Boisset et al; Kissa & Herbomel; Lam et al, see Klaus & Robin 2017). The breakthrough was possible owing to the power of technical imaging approaches and in particular confocal time-lapse fluorescent microscopy on the living animal, more specifically the zebrafish embryo. This model organism offers unique advantages owing to its small size, its fast development and, last but not least, its transparency. This latter advantage offers unique opportunities for in-depth live imaging and was essential to achieve the goal that we had in the mind when we started our recently published work; i.e imaging the emergence of hematopoietic stem cells from the aortic wall at sufficient spatio-temporal resolution to capture potentially short-lived events and critical intermediate stages so as to be able to propose a comprehensive model of the EHT. In addition, we intended to perform an in–depth descriptive study of the sequential steps of the EHT without disconnecting it from its natural environment. Hence, we aimed at visualizing the morphodynamic changes of endothelial cells, meaning the whole aortic landscape surrounding hemogenic regions, concomitantly to the EHT. The rationale behind this is that some of the EHT unique features when the process is compared with any other cellular dynamic event leading to the extrusion of a cell from an organized epithelium (among which the very peculiar bending of the emerging cell, primarily visualized in Kissa & Herbomel 2010, see also figure 1), suggested that it is adapted to the biomechanical properties of the aortic wall, made of very flat endothelial cells subjected to high mechanical load and exposed to the multidirectional forces exerted by the blood flow. Beyond this and on more conceptual grounds stands also the fundamental issue of the influence of the blood flow on the fate of hemogenic and hematopoietic stem cells (see also later).
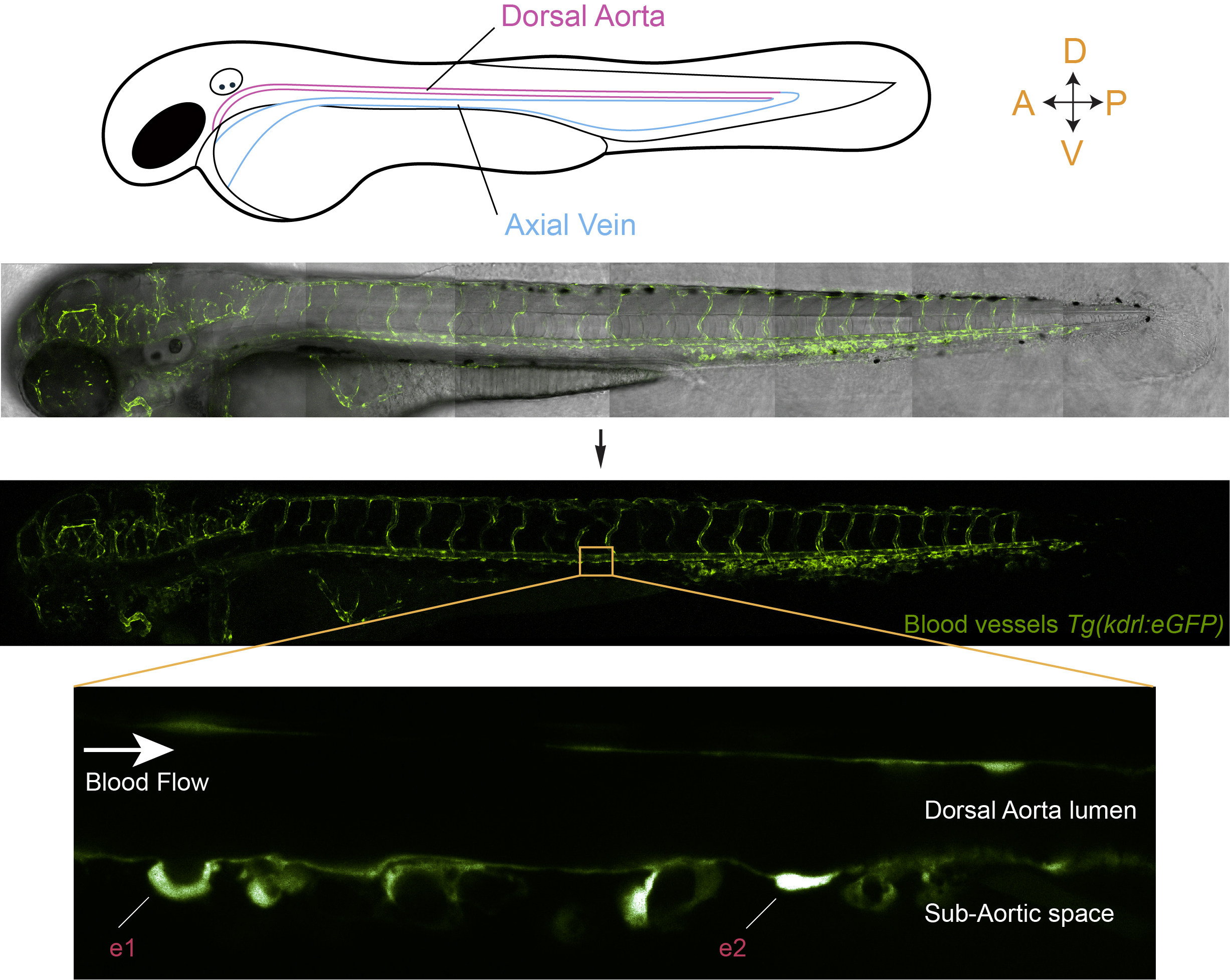
Figure 1: In vivo visualization of hematopoietic stem cell emergence via the EHT, from the aortic floor, in a live zebrafish embryo. The middle panels of the figure show the organization of the vascular system in a transgenic line that expresses eGFP under the control of a vascular promoter. The bottom panel shows a magnification of a region of the dorsal aorta in the trunk of the embryo and highlights the emergence of hematopoietic cells via the EHT (e1 and e2; e1 is a more advanced stage than e2).
Let’s come back to the work now and share with the reader our motivation to invest time and energy on the project as well as telling about its experimental challenges.
When Mylène started her PhD, she did not have a strong technical expertise on imaging approaches but was already well acquainted with the zebrafish model. She undertook however the challenge of imaging the EHT more in depth “I was fascinated by the way hematopoietic stem cells are born. I was also really determined to find out the most appropriate set up to visualize as clearly as possible the dynamics of intracellular components during the EHT within the live embryo. Here began a very long journey during which I learned from many imaging specialists and started to realize how tricky in vivo imaging is“, as she said. From my side, when I started with this topic, about a year after Mylène did, with a strong expertise in cell biology and biological membrane dynamics as well as a strong motivation to modelling the EHT, we joined forces and started to analyze the series of time-lapse sequences that she had accumulated in the meanwhile and that were aimed at focusing on two aspects of cell biological features of EHT undergoing cells: the dynamics of their luminal and basal membranes as well as intracellular actin organization (by using double transgenic fish line expressing the membrane marker ras-mCherry and the actin reported Lifeact-eGFP, respectively). But looking into the movies made us realize that few of them would actually allow visualizing the entire EHT process with enough resolution (in particular for the sealing of the aortic floor and the release steps) and be able to reproduce several times each significant observation. This is because we had to face a series of major issues: (i) the stochastic initiation of the EHT (EHT cells are born from a sub-population of endothelial cells belonging to the so-called hemogenic endothelium but you never know if and when a cell will initiate the EHT; to circumvent the problem, we often started imaging when the cell had already begun its typical bending (which we refer in our work as to the cup-shaped stage)), (ii) the stochastic length of the process (that varies very much, ranging from approximately 4 to more than 15 hours, hence raising the issue of phototoxicity after long periods of laser exposure, see our paper for more details), (iii) the growth of the embryos that triggers drifts as well as the movements associated with the beating of the heart. We also had to fight against mosaicism (not all hemogenic regions of the aortic floor were expressing the fluorescent marker of interest, thus decreasing the chance to image the relevant events successfully). Mosaicism hampered quite significantly the work when using transient transgenesis or another fish line that we had wished to use to follow intercellular contacts between EHT undergoing cells and their endothelial neighbours (and that finally brought us most valuable information; a transgenic line that expresses the junctional protein eGFP-ZO1 that Mylène had managed to obtain, after several rounds of selection (meaning 2 years of efforts to select the best expressing fishes)). So, on several occasions when the project was developing we have been thinking that the outcome of the fastidious work should better be worth the effort !!! At the end, when we made the final selection of time-lapses that would constitute the body of the publishable work, we used less then a 100 of them and had collected more than 500 over 4 years (which makes …. 5 TB to scrutinize !).
Apart from the visualization of the EHT sequential steps and key features, as mentioned above, our aim was also to picture the EHT in the developmental vascular landscape. This is not a simple issue if one wants to get a clear picture because during the EHT time-window (that lasts from approximately 30 hrs post-fertilization (hpf) to 60 hpf), the aorta shrinks in diameter, must adapt to the loss of cells constituting the hemogenic endothelium and aortic cells undergo cell-shape changes as they elongate to adapt to mechanical tension (Lagendijk et al 2017). To standardize as much as possible the analysis, we started imaging at 48 hpf (which is also the timing at which the EHT is culminating). When one looks through the Z-stacks of an acquisition, even with a 3D-rendering view, one realizes that our brain experiences difficulties embodying the information and morphometrics becomes quite tricky. Things become even more difficult if one wants to explore the dynamics and inter-relation of objects through time, which is what we wanted to do. To fulfil our aim, we initiated an essential collaboration with experts in image analysis and physics from the Pasteur campus, namely Jean-Yves Tinevez and Fabrice de Chaumont. This interdisciplinary collaboration was most fruitful. The team developed an algorithm capable of deploying the aortic wall to project the fluorescent signals onto a 2D-plane (see figure 2) and of accurately re-iterating the aortic projections through time (taking into account distortions of the aorta and its morphological changes). With this algorithm, we have been able to exploit several of our time-lapse sequences and in particular the ones performed with eGFP-ZO1 expressing fishes that allowed following the cell boundaries and inter-cellular contacts. With this, we managed to visualize unambiguously the symmetric division of EHT undergoing cells (an amazing event when you think about the tension that must be exerted on the emerging cells !) as well as the dynamic interplay between EHT cells and their endothelial neighbours. We observed that the number of endothelial cells contacting EHT ones decrease with time, most probably to minimize the risk of leakage upon sealing of the endothelium. Importantly, the clear dynamic views provided by the 2D-projections revealed that the cells from the hemogenic endothelium, around 48 hpf, are rather elongated (in comparison to the surrounding endothelial cells) and that progression of the emergence is characterized by the contraction of the interface with neighbours that proceeds along the antero-posterior axis (the interface delimitating the EHT cell apex facing the aortic lumen). This axis parallels the blood flow, raising in our mind the idea that the direction of the contraction, anisotropic since oriented preferentially along a specific axis, may be minimizing the exposure to hemodynamic forces. Finally, the 2D-mapping of our time-lapse sequences performed on embryos expressing the actin reporter Lifeact also revealed anisotropy in the organization of sub-cortical actin, with its densification at sub-plasmalemmal regions of hemogenic and EHT cells enriched in junctional proteins. This suggested that these regions (whose orientation is perpendicular to the blood flow) are the most exposed to mechanical tension. These interpretations are now awaiting being tackled by further modelling of the forces at play.
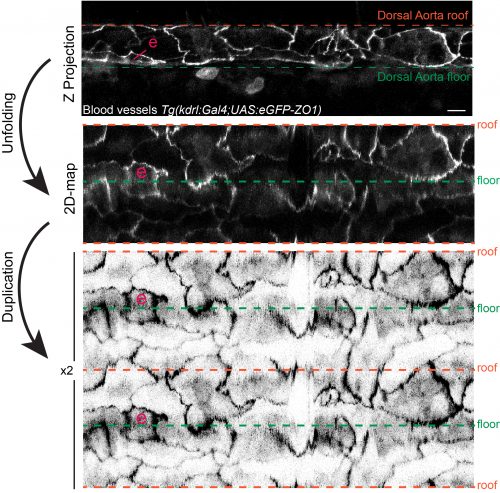
Figure 2: 2D-map representation of the dorsal aorta after its deployment using the TubeSkinner plugin of the ICY software. “e” highlights a cell undergoing the EHT. The transgenic fish expresses eGFP-ZO1, ZO1 being part of the complex building the tight junctions. For more details on the 2D-algorithm see our paper (https://doi.org/10.7554/eLife.37355).
Overall, our results strongly suggest that anisotropy, because it organizes according to the blood flow axis, may be dictated by the mechanical constraints imposed by the aortic environment and in particular the blood flow. Thus, what would happen if blood flow is inhibited from the very beginning ? To answer to this question, we prevented blood flow by blocking heart beating (amazingly, the zebrafish embryo can survive several days without heart beat and circulation, by passive diffusion of gas through the skin). In this situation, we observed the impairment of actin cytoskeleton anisotropic organization and destabilization of junctional complexes. Quite surprisingly, hemogenic cells were still capable of escaping from the aortic wall (with, surprisingly also, the apparent maintenance of their localization on the ventral side of the aorta). However, they managed to do so both toward the sub-aortic space – as they do under normal physiological conditions – and the aortic lumen. The intra-aortic emergence most probably took place because the force that normally applies to the aortic wall and that is perpendicular to the arterial axis (the so-called mechanical strain) does not act anymore to push the emergence toward the sub-aortic space. Hence, even in the absence of blood flow, a population of aortic cells retains the ability to escape from the endothelium. This was unexpected because the emergence of hematopoietic stem cells was shown to depend on the activation of a transcription factor essential for hematopoiesis and whose expression is induced by the blood flow (Runx1, see Adamo et al 2009; see also for a seminal paper on the influence of blood flow on hematopoiesis North et al 2009). However, we do not know if those cells, released in the absence of blood flow, do retain the bona fide properties of hematopoietic stem cells, meaning the capacity to differentiate and replenish the entire repertoire of immune cells of the adult body. It is probable that they don’t and that they will end up dying, which is what we observed for some of them, few hours after the release. What these results suggest in addition is that, even if essential pathways are impaired, cells programmed to become hematopoietic may retain some of their abilities, such as escaping from the endothelial environment. This is possibly the reason why in vitro settings aimed at producing hematopoietic stem cells for regenerative purposes and using pluripotent stem cells produce hematopoietic-like cells that ultimately fail to express full hematopoietic potential. Indeed, hematopoietic stem cells are among the rare ones that cannot be produced yet and our work reinforces the idea that the mechanical constraints of the aortic environment are required to produce bona fide hematopoietic stem cells. It would be very interesting in the future to address the question as of the influence, on the fate of hematopoietic stem cells, of mechanical forces taking place contemporarily to the emergence.
So, at the end, our investment was really worth the effort and we gleaned interesting results and ideas from the hundreds of time-lapse sequences that were accumulated by Mylène during her PhD. Currently, we hope that our work, beside its contribution to the understanding of the Cell Biology and Biomechanics of cell extrusion processes, will bring valuable knowledge for reproducing at will the genesis of hematopoietic stem cells.
Anne Schmidt & Mylène Lancino
Developmental and Stem Cell Biology Department
CNRS UMR3738
Macrophages and Development of Immunity
INSTITUT PASTEUR
25 rue du Dr. Roux
75724 Paris Cedex 15 France
anne.schmidt@pasteur.fr
mylène.lancino@pasteur.fr
_______________
References
Adamo, L., O. Naveiras, P. L. Wenzel, S. McKinney-Freeman, P. J. Mack, J. Gracia-Sancho, A. Suchy-Dicey, M. Yoshimoto, M. W. Lensch, M. C. Yoder, G. Garcia-Cardena, and G. Q. Daley.2009. Biomechanical forces promote embryonic haematopoiesis. Nature 459:1131-5.
Bertrand, J. Y., N. C. Chi, B. Santoso, S. Teng, D. Y. Stainier, and D. Traver.2010. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464:108-11.
Boisset, J.C., van Cappellen, W., Andrieu-Soler, C., Galjart, N., Dzierzak, E., and Robin, C.2010. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature464, 116-120.
Jordan, H. E.1917. Aortic Cell Clusters in Vertebrate Embryos. Proc Natl Acad Sci U S A 3:149-56.
Kissa, K., and P. Herbomel.2010. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464:112-5.
Klaus, A., and C. Robin.2017. Embryonic hematopoiesis under microscopic observation. Dev Biol 428:318-327.
Lagendijk, A. K., G. A. Gomez, S. Baek, D. Hesselson, W. E. Hughes, S. Paterson, D. E. Conway, H. G. Belting, M. Affolter, K. A. Smith, M. A. Schwartz, A. S. Yap, and B. M. Hogan.2017. Live imaging molecular changes in junctional tension upon VE-cadherin in zebrafish. Nat Commun 8:1402.
Lam, E. Y., C. J. Hall, P. S. Crosier, K. E. Crosier, and M. V. Flores.2010. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood 116:909-14.
North, T. E., W. Goessling, M. Peeters, P. Li, C. Ceol, A. M. Lord, G. J. Weber, J. Harris, C. C. Cutting, P. Huang, E. Dzierzak, and L. I. Zon.2009. Hematopoietic stem cell development is dependent on blood flow. Cell 137:736-48.
Sabin FR.1917. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. Anat. Rec. 13:199-204.


 (3 votes)
(3 votes)