Ascidian embryonic cells offer clues to the evolutionary origin of vertebrate neural crest cells and neuromesodermal progenitors
Posted by Tasuku Ishida, on 11 June 2024
Vertebrates, including humans, possess a “head” comprising cranial bones, the central nervous system, and sensory organs. It is believed that the emergence of the vertebrate “new head” is closely linked with the evolutionary acquisition of two cell populations: neural crest cells (NCCs) and cranial placode cells. Therefore, understanding the evolutionary origin and history of NCCs and cranial placode cells is crucial for understanding the evolution of vertebrates.
In vertebrate embryos, both NCCs and cranial placode cells arise from the border region between the neural plate and the epidermis. NCCs are unique because they produce not only cell types of ectodermal origin, such as sensory neurons and melanocytes, but also cell types of mesodermal origin, including smooth muscle cells, osteocytes, and chondrocytes.
Ascidians: fascinating model organisms for evolutionary developmental biology
Ascidians, commonly known as sea squirts, belong to the subphylum Urochordata or Tunicata, the sister group of vertebrates. They have been providing key insights into chordate developmental mechanisms and their evolution (FIGURE 1). Recent studies suggested that ascidian embryos have cells that share an evolutionary origin with vertebrate NCCs[1-3]. For example, ascidian cells called a9.49, located in the neural plate border, likely share an evolutionary origin with vertebrate NCCs[1]. Indeed, this cell pair expresses orthologous genes that specify the neural plate border cells and NCCs in vertebrate embryos. Furthermore, a9.49 cells can be reprogrammed to migratory pigment cells by overexpression of Twist, which encodes a transcription factor for mesenchyme specification. However, unlike vertebrate NCCs, ascidian NCC-like cells identified thus far do not produce cell types that are commonly of mesodermal origin. Therefore, it is believed that the multipotency of NCCs has been acquired within the vertebrate lineage after the split from the ascidian lineage.
A key observation made nearly 40 years ago
In 1987, Nishida found that ascidian cells called b8.17 and b8.19 give rise to muscle cells, nerve cord cells, and endodermal cells near the tip of the tail of embryos[4]. Both b8.17 cells and b8.19 cells are located in the neural plate border, which abuts the neural plate cells that give rise to the central nervous system. These cells express many orthologous genes that specify the neural plate border cells and NCCs in vertebrates. Therefore, if b8.17 and b8.19 cells share an evolutionary origin with vertebrate NCCs and produce cell types that are commonly ectodermal and mesodermal origin, the potential of NCCs to produce cells of multiple germ layers may date back to the last common ancestor (LCA) of vertebrates and ascidians, contrary to the prevailing hypothesis explained above.
In light of this context, we have decided to investigate the possibility that ascidian b8.17 and b8.19 cells share an evolutionary origin with vertebrate NCCs. First, we confirmed that b8.17 cells indeed produced muscle cells, as Nishida showed previously[4]. Second, we showed that these ascidian cells expressed Msx, Zic, Pax3/7, and Snai, which encode orthologs of key transcriptional factors specifying neural plate border cells of vertebrate embryos. We indeed showed that these genes were involved in specifying these ascidian cells. The location and the gene circuit for specification indicate that this ascidian cell population shares an evolutionary origin with vertebrate NCCs.
Do neural plate border cells of ascidian embryos share the evolutionary origin with vertebrate neuromesodermal progenitors (NMPs)?
In the middle gastrula stage, the ascidian neural plate border consists of four cells: b9.34, b9.33, b9.37, and b9.38, in order from posterior to anterior. In later embryos, the anterior two cells (b9.37 and b9.38) give rise to nerve cord cells (commonly of ectodermal origin), and the posterior two cells (b9.34 and b9.33) give rise to muscle cells (commonly of mesodermal origin) and other cells near the tip of the tail region[4]. On the basis of this observation, we hypothesized that these cells may share an evolutionary origin with vertebrate neuromesodermal progenitors (NMPs).
In vertebrates, Tbx6 is expressed in NMP-derived mesodermal cells and Tbx6 negatively regulates Sox2, which is expressed in NMP-derived spinal cord cells[5]. If our hypothesis is correct, the gene regulatory circuit consisting of Tbx6 (or its orthologs) and Sox2 (or its orthologs) will also be used for fate decisions in the neural plate border cells of ascidian embryos. Indeed, the anterior cells, which give rise to the nerve cord, expressed Sox2 ortholog (Sox1/2/3), and the posterior cells, which give rise to muscle, expressed Tbx6 ortholog (Tbx6-related) (FIGURE 2). Overexpression of Tbx6-related downregulated Sox1/2/3, and promoted muscle fate. Thus, the ascidian neural plate border cells and vertebrate NMPs share the gene regulatory circuit of Sox2 and Tbx6. In addition, a comparative single-cell transcriptome analysis also supported a close relationship between these ascidian cells and NMPs of zebrafish embryos.
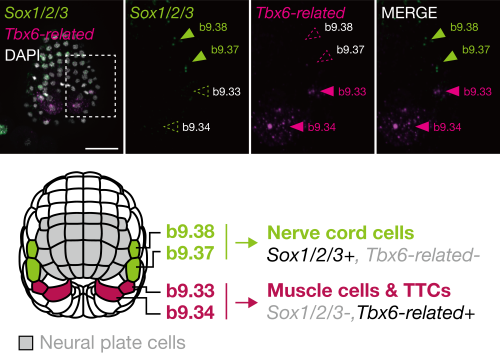
In this way, this ascidian cell population has properties of both vertebrate NMPs and NCCs. Therefore, the LCA of tunicates and vertebrates likely had cells with a hybrid property of NCCs and NMPs, and such ancestral cells may have produced both ectodermal and mesodermal cells.
Chordate origin of NCCs and NMPs
A logical follow-up question to ask is whether the Cephalochordata, the sister group of Olfactores, possessed NCC-like cells and NMP-like cells. Cephalochordates, commonly known as lancelets or amphioxus, are filter-feeding marine animals and are believed to retain ancestral features of chordates. Amphioxus is believed to lack cells homologous to vertebrate NCCs[6], although a recent preprint indicated that amphioxus possesses migratory NCC-like cells[7].
Interestingly, somites, notochord cells, dorsal neural tube, and hindgut of the posterior part of amphioxus embryos are produced from a cell population near the tip of the tail[8]. Therefore, amphioxus may possess NMP-like cells. Elucidating the developmental mechanism of this cell population should shed light on the evolution of the body plan of chordates.
A possible evolutionary history of the stemness of NCCs/NMPs
The ascidian NCCs/NMP-like cell population does not possess stemness: they do not show the ability of self-renewal, although they produce cell types that are commonly ectoderm and mesoderm origin. In vertebrates, the high stem cell-like potential of NCCs may depend on pluripotent factors or Yamanaka factors[9-12]. Among Yamanaka factor genes, only Sox1/2/3 was known to be expressed in the ascidian NCCs/NMP-like cells. This may be a reason why the ascidian cells do not have self-renewal ability.
Altogether, we propose a two-step model for the evolution of stemness of NCCs/NMPs: 1) the ability to produce ectodermal and mesodermal cells came first, and 2) the self-renewal ability, which led to acquisition of bona fide NCCs and NMPs. Future works on non-ascidian tunicates (e.g., Oikopleura), amphioxus, and cyclostomes will shed light on the evolution of the stemness of NCCs/NMPs. It would be particularly important to associate the evolution of the stemness of NCCs/NMPs with the evolutionary acquisition of pluripotent factor genes and whole genome duplications that occurred in the vertebrate lineages.
References


 (4 votes)
(4 votes)