Behind the paper: Characterising the spatiotemporal dynamics of microglia across the human lifespan
Posted by David Menassa, on 17 October 2022
Dr David A. Menassa and Professor Diego Gomez-Nicola at the University of Southampton, UK recently published an article in Developmental Cell where they reveal how the microglial population colonises the human frontal cortex. Dr Menassa gave us a behind the scenes look at how the story came together.
- How did you get started on this project?
I joined the Gomez-Nicola lab in October 2017 as a research fellow. The funding had been granted by the Leverhulme Trust prior to my arrival, and all was in place to set the human lifespan study in motion.
The task of securing healthy human tissues from whole embryos to advanced ageing was a challenging one. It took several years to establish the tissue collection and 12 tissue resources within the UK and abroad were consulted. There was a substantial administrative load. Human tissue work in general is fraught with challenges: even when one acquires the tissues, there are no guarantees that the antigenic targets will have been preserved. The assumption is that they should be, but there are many factors that come into play making work with human tissues a good exercise in patience, focus and resilience. Once the frustrations were out of the way, the project kicked off at full speed. I was lucky to be part of a very supportive laboratory environment, working alongside a team of intramural and extramural collaborators. This paper is evidence of great teamwork and we are delighted to be able to share our findings with the scientific community.
- What was already known about the developmental dynamics of microglia in the human brain prior to your work?
Two studies on adult human tissues (including one from the Gomez-Nicola lab) were published in 2017 showing that microglial turnover in humans is much faster than previously thought and that the entire population is renewed several times during a lifetime [2, 3]. Additionally, microglial proliferative dynamics increase during pathology and have been the target of therapies to limit proliferation in neurodegenerative disease particularly Alzheimer’s disease models [4]. Very little was known about microglial dynamics during the critical stages of human brain development and the early postnatal age, and this was largely due to the limited availability of tissues for research.
In the latter half of 2020, two papers profiled microglial developmental states during early embryonic and early fetal lives in humans [5, 6]. These studies showed that ontogenic pathways were conserved between mice and humans and that microglia became immunocompetent from as early as the 11th week of gestation. In 2022, the regional microglial transcriptome was described in humans between 8-23 weeks of gestation: this was the first study to look at specific anatomical regions such as the cortex and the cerebellum [7]. Altogether, these studies offered an important view of specific temporal windows during development. In our study, we covered the entire human lifespan, detailing the spatiotemporal dynamics of microglia from the moment these cells arrive to the human developing brain at 4 pcw up until they turnover very slowly in adult life. We profiled these cells in all layers of the cortex in the frontal lobe, the brain region responsible for higher order cognitive and executive function in humans.
- Can you summarise your findings?
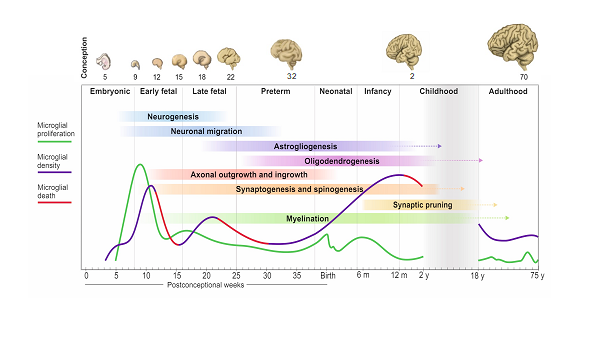
There are four important temporal windows during human brain development when we see microglial changes: the first one is when microglia arrive to the brain with the onset of circulation at 4 pcw; the second is at the transition between embryonic to fetal life at 9-10 pcw; the third is at 13-14 pcw and the fourth is during early childhood between 0.5-1 year of age. Microglial expansion in the brain is via a wave-like pattern of cycles of migration and proliferation, increasing cell number, which is further refined by selective cell-death. The changes in microglial dynamics are aligned with co-occurring neurodevelopmental processes. We found no sex differences during these developmental stages. In adult life, the population self-renews at a relatively steady rate until advanced ageing. This pattern of colonisation is unique to humans and is strikingly different than what we see in mouse.
- When doing the research, did you have any particular result or eureka moment that has stuck with you?
Seeing the first fully labelled embryo for microglia, other tissue-resident macrophages and proliferative cells in the brain matter, liver, spleen, spinal cord, and heart all visible in one slide was a special moment. I remember sending Dr Gomez-Nicola the first image and we were both excited and thrilled that it was all worth the wait; the signal was very clear and from that point onwards, we were confident that the project was heading in the right direction. The second moment was when we had all the data unblinded and plotted across the lifespan for all tissues: it was a roadmap, and we could see the exact pattern that microglia followed prenatally and postnatally.
- And what about the flipside? Any moment of frustration or despair?
Human tissue research is challenging, but the end-result is very rewarding. In this paper, we could not obtain samples with consent for research use from late childhood and adolescence. Therefore, we are missing part of the whole picture. As the pattern of growth and myelination of the frontal lobe is unique in humans during these stages, it will be interesting to see what happens to microglial dynamics.
- Where will this story take you next?
Now that we know the temporal windows of relevance in microglial dynamics, the next step is to begin dissecting how microglial cells interact with the neurodevelopmental environment. This could be by elucidating the cues that these cells get from the brain at each step and in this way, we can start identifying why the population behaves the way it does. This is important as microglia are part of the neuropathology of neurodevelopmental disorders. To find out how these cells contribute to normal and altered neurodevelopment, we need to dissect the signalling pathways between them and developing neurons.
- What is next for you after this paper?
I am currently stipendiary lecturer of neurophysiology and neuroscience at the Queen’s College, University of Oxford and visiting researcher in neurodevelopment at the Croatian Institute for Brain Research, University of Zagreb. I am continuing my research on microglial development and how this relates to neurodevelopmental disorders. I am applying for tenured positions in the UK and Europe to set up my own research group.
Bibliography
1. Menassa, D.A., et al., The spatiotemporal dynamics of microglia across the human lifespan. Dev Cell, 2022.
2. Askew, K., et al., Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep, 2017. 18(2): p. 391-405.
3. Réu, P., et al., The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep, 2017. 20(4): p. 779-784.
4. Olmos-Alonso, A., et al., Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain, 2016. 139(Pt 3): p. 891-907.
5. Bian, Z., et al., Deciphering human macrophage development at single-cell resolution. Nature, 2020. 582(7813): p. 571-576.
6. Kracht, L., et al., Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science, 2020. 369(6503): p. 530-537.
7. Li, Y., et al., Decoding the temporal and regional specification of microglia in the developing human brain. Cell Stem Cell, 2022. 29(4): p. 620-634.e6.


 (1 votes)
(1 votes)