BSDB Gurdon Summer Studentship Report (20)
Posted by BSDB, on 4 December 2017
![]() Established by the British Society for Developmental Biology in 2014, The Gurdon/The Company of Biologists Summer Studentship scheme provides financial support to allow highly motivated undergraduate students an opportunity to engage in practical research during their summer vacation. Each year, ten successful applicants spend eight weeks in the research laboratories of their choices, and the feedback we receive is outstanding.
Established by the British Society for Developmental Biology in 2014, The Gurdon/The Company of Biologists Summer Studentship scheme provides financial support to allow highly motivated undergraduate students an opportunity to engage in practical research during their summer vacation. Each year, ten successful applicants spend eight weeks in the research laboratories of their choices, and the feedback we receive is outstanding.
Our ninth report from the 2017 group of student awardees comes from Miguel Robles Garcia (student at The University of East Anglia), who undertook his studentship with Andrea Münsterberg at The University of East Anglia.
During vertebrate development, the heart is one of the first organs to develop. It is known that during this process many malformations can occur, which are capable of affecting correct heart function leading to potential defects or death. The developmental stages between mammals and birds are similar starting with cardiac looping and resulting in chamber formation. Due to the importance of the heart, its development is tightly regulated by different transcriptional and post transcriptional signalling pathways driven by transcription factors and microRNAs (miRs) respectively. miRs are regulators of gene expression that inhibit the translation of mRNA. During this project, my focus was to aid the postdoctoral researcher with her investigation of miR-133 function, which is thought to regulate BAF60b chromatin during heart development.
I started my time in the lab by dissecting wild type (WT) embryos from stages HH18-19 and HH23-25. The staging of the chick development follows the one described by Hamburger and Hamilton from 1951. Throughout my time in the laboratory, I became familiar with the dissection technique in which the different membranes that cover the embryo need to be removed. This is needed so that in future analyses (such as those that are mentioned) the membranes would not infer in the procedure and to make the tissue more available. My project then shifted towards the injection of embryos at stages HH 14-16 with the antagomir-133 (AM133). An antagomir is a modified oligonucleotide that binds to its specific micro RNA and inhibits its action. Therefore, this technique can be used to investigate potential effects in hearts which are deprived of a specific micro RNA. I used AM133 which is designed to inhibit miR-133. I also used scrambeled antagomiR (SCR) as a control for the procedure to see that the actual way of injecting the oligonucleotide wasn’t the one affecting the development of the heart. Both of these oligonucleotides have 5’ fluorescent label that allows the injection to be seen at first with the naked eye, but later after further incubation, with the aid of a GFP microscope (Figure 1). These antagomiRs were injected into a hollow cavity close to the heart walls while the heart is still beating without killing the embryo. After
the injection, the embryos were sealed with tape and returned to an incubator at 37°C for 24/48h depending on the intended analysis.
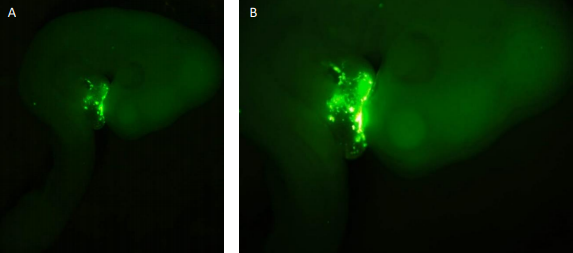
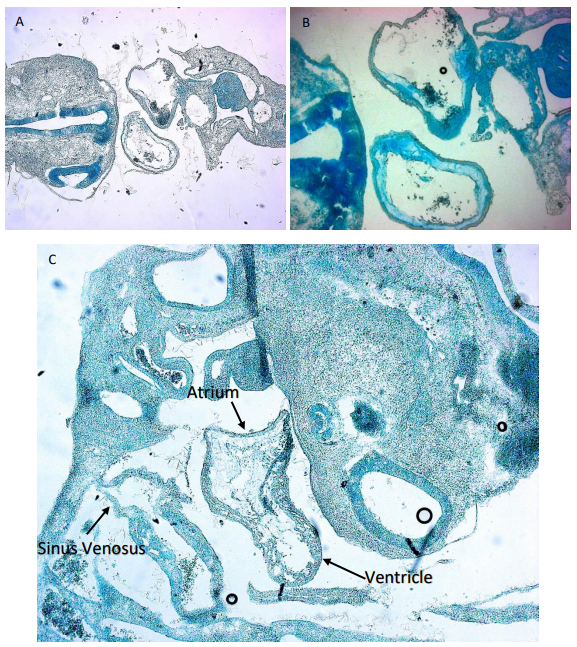
While some of the embryos were used for imaging and making sure the inhibitor bound in the correct place as shown in Figure 1, other embryos were embedded for sectioning. This involved placing the embryos in a gelatine medium that would then solidify at room temperature. Two different approaches were used for this in which one meant leaving the embedded embryos at room temperature for the gelatine to solidify slowly while the other was performed using dry ice for a rapid solidification. This allowed me to section the embryos for a closer examination of the heart. SCR embryos were compared to AM133 injected embryos to see if there is any difference in morphology. However, in order to image the slides containing the embryo section I had to stain them. For this we used an Alcian blue staining procedure (Figure 2). In this case the medium surrounding the tissues of the embryos would be discarded and only the glycosaminoglycans within the actual tissue were stained blue. This was also performed for embryos that had a double knock out of miR133 and miR1.
I want to thank the BSDB Gurdon Studentship for granting me the opportunity to have what I can just describe as an incredible experience. It has allowed me to develop my skills and scientific mind which I will be able to apply in my future studies and career. This opportunity is of great importance as, as a third-year student, I wasn’t sure of how daily lab teamwork alongside other scientists that are dedicated to their research felt like. I would also like to thank the Münsterberg lab for hosting me and Estefanía and Johannes for their guidance through these weeks.


 (No Ratings Yet)
(No Ratings Yet)