BSDB Gurdon/The Company of Biologists 2019 Summer Studentship Report – Matyas Bubna
Posted by BSDB, on 19 January 2020
Established by the British Society for Developmental Biology in 2014, The Gurdon/The Company of Biologists Summer Studentship scheme provides financial support to allow highly motivated undergraduate students an opportunity to engage in practical research during their summer vacation. Each year, ten successful applicants spend eight weeks in the research laboratories of their choices, and the feedback we receive is outstanding. You can read accounts from previous years here. If you’re interested in applying or hosting a student in 2020, applications need to be in by the end of March.
Our sixth report from the class of 2019 comes from Matyas Bubna (QMUL) who studied frog neural crest in Roberto Mayor’s lab at UCL.
Visualising neural crest induction, migration and differentiation in Xenopus
Throughout my undergraduate studies I have become increasingly captivated by the intricacy and elegance of animal development. Especially interesting to me is how processes such as morphogenesis, tissue patterning or cell migration, which can appear incomprehensible, emerge from relatively simple interactions at the molecular level. Observing how a single cell transforms into a complex organism is a unique and thought-provoking experience. I am grateful to the BSDB for the studentship allowing me to explore this field. I would strongly recommend the Gurdon studentship to anyone interested in topics ranging from evolution, epigenetics, cell signalling, cancer, to stem cells and regeneration – all of these processes may be elucidated by taking a developmental point of view.
During the summer studentship I have learnt to work with Xenopus laevis embryos and keep track of their development. The aim of my project was to optimise techniques for visualising the neural crest with other tissues relevant to its induction, migration and differentiation. To do this I used two well established techniques in developmental biology: in situ hybridisation and immunofluorescence (see Figure 1).
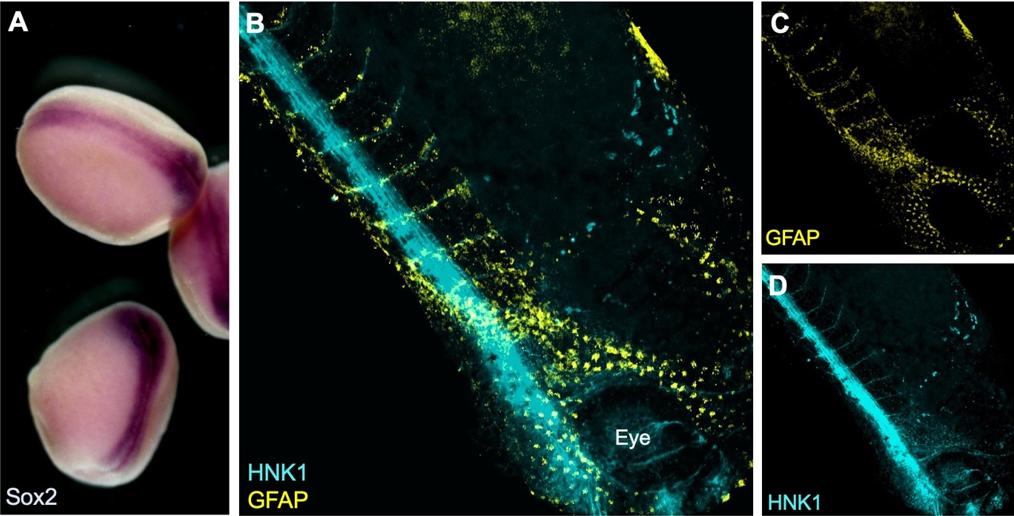
Why study neural crest cells?
The neural crest is a fascinating population of cells unique to vertebrates, which is induced at the neural plate border and following neurulation delaminates and migrates away from the neural tube. A subset of these cells migrates into head regions where it gives rise to a variety of tissues including bones, cartilage, as well as neurons. This cranial neural crest also migrates into the branchial (or pharyngeal) arches and contributes in a major part to the craniofacial skeleton. This has undergone major changes during vertebrate evolution. For instance, some of the jaw bones present in our common ancestors with reptiles have given rise to the middle ear bones of mammals (Santagati & Rijli, 2003). A possible explanation for this versatility of the neural crest is that its cells retain multipotency for longer than the three embryonic germ layers, prompting some to consider it a ‘fourth germ layer’ (Simoes-Costa & Bronner, 2015).
The diversity of neural crest-derived cells, which also includes melanocytes, Schwann cells, meninges or the cornea, makes it an important model of differentiation with a potential for therapeutic applications. It is also a great model for studying cell migration (Szabó & Mayor, 2018) and by proxy epithelial to mesenchymal transition in cancer metastasis. Recent work even attributed the ability to remove cellular debris from early neural tube to migrating neural crest cells, potentially through a macrophage-like mechanism (Zhu, et al., 2019).
Going further with established techniques
I have been testing and optimising techniques to visualise proteins and gene expression that may help elucidate how the neural crest gets induced and how it migrates through the embryo. For example, I have image the neural crest with nearby mesoderm, which is required during induction, using a combination of fluorescent in situ hybridisation (FISH) and immunostaining. In order to visualise expression of two different genes within the embryo a double in situ hybridisation (ISH) can be used. This is especially important in Xenopus, as no antibody is known to efficiently and exclusively label the neural crest. Although colorimetric ISH is easier and does not require clearing of the tissues, it doesn’t enable exploring the 3D structure by imaging the whole embryo at once. I have used confocal and multiphoton microscopy to analyse the embryos (see Figure 2). Unfortunately, it seems that using two RNA probes at the same time reduces signal intensity and although I have tried to optimise the signal amplification reaction and bleaching to neutralise endogenous peroxidase activity, I have not been able to reduce the background.
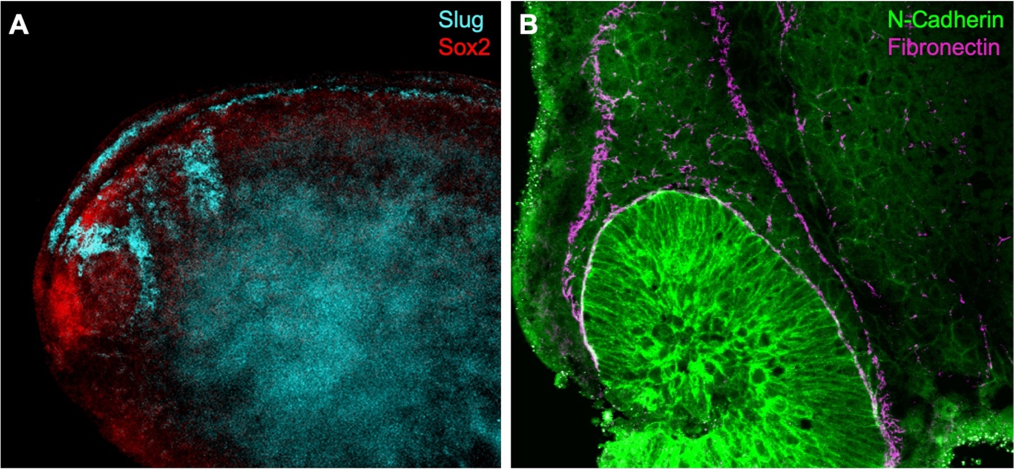
One possibility is to delimit the neural crest using an antibody for fibronectin, an extracellular matrix component that encourages neural crest migration. Using double immunostaining (Figure 2B) I was able to confirm migratory neural crest cells express N-Cadherin near the optic vesicle as fibronectin outlines the neural crest streams (Scarpa, et al., 2015). Towards the end of my summer project, I have also manipulated cell contractility using drug treatments to observe changes in neural crest cell behaviour using several antibodies.
I thank Prof Roberto Mayor for supervising this summer project and Dr Adam Shellard for teaching me methods used in the lab. I have learnt a lot about how research is done and presented my results at a lab meeting in the final week and used this experience to transition from undergraduate study into my PhD.
References
- Santagati, F. & Rijli, F. M., 2003. Cranial neural crest and the building of the vertebrate head. Nature Reviews Neuroscience, Issue 4, p. 806–818.
- Scarpa, E. et al., 2015. Cadherin Switch during EMT in Neural Crest Cells Leads to Contact Inhibition of Locomotion via Repolarization of Forces. 34(4), pp. 421-434.
- Simoes-Costa, M. & Bronner, M. E., 2015. Establishing neural crest identity: a gene regulatory recipe. Development, Volume 142, pp. 242-257.
- Szabó, A. & Mayor, R., 2018. Mechanisms of Neural Crest Migration. Annual Review of Genetics , Volume 52, pp. 43-63.
- Zhu, Y. et al., 2019. Migratory Neural Crest Cells Phagocytose Dead Cells in the Developing Nervous System. Cell, 179(1), pp. 74-89.



 (No Ratings Yet)
(No Ratings Yet)