Cellular Senescence in Regeneration
Posted by Max_Yun, on 28 June 2015
Salamanders are remarkable organisms. Following the amputation or loss of complex structures such as parts of their eyes, hearts and brains, tails -including the spinal cord-, jaws and even full limbs, they are able to set up a regeneration programme which leads to the exact replacement of the missing structure, even as adults. As such, salamanders are considered the champions of regeneration. And, if the ability to regrow a full limb during adulthood was not surprising enough, they are able to do this over and over again, as their regenerative abilities do not decline as they age -unlike in most other organisms, including humans. This notable characteristic of salamander regeneration was recently demonstrated by an elegant experiment carried out by Goro Eguchi, Panagiotis Tsonis and co-workers1, who systematically removed the lens of a cohort of newts 18 times during 16 years and showed, in a nutshell, that the resulting lens was almost identical to a young lens that has never regenerated. Hence, the regeneration process in these organisms can withstand the test of time. But, how is this possible? I recently set out to address this question and found that the answer may lie in a highly efficient mechanism of eliminating senescent cells2.
Cellular senescence is a process by which cells that have been exposed to various types of DNA damage, telomere erosion and oncogenic stresses undergo a permanent withdrawal from the cell cycle. Like apoptosis, this cellular mechanism prevents the transmission of damaged genetic material, constituting a powerful anti-tumourigenic strategy. Unlike apoptosis however, it does not lead to cell death but to cells which are metabolically active and that can modify their microenvironment -through the acquisition of a senescence-associated secretory phenotype comprising growth factors, cytokines and matrix-remodelling proteins-, sometimes for the worse. Indeed, in recent years it has become clear that senescent cells can have detrimental effects on biological processes. This is particularly relevant to Ageing, as most organisms accumulate senescent cells during their lifetime, leading to a number of age-related disorders3. More recently, cellular senescence has also been linked to the age-related decline of regenerative abilities seen in mammals4. Given that such decline is not seen in salamanders, could it be possible that these organisms possess mechanisms to curtail cellular senescence?
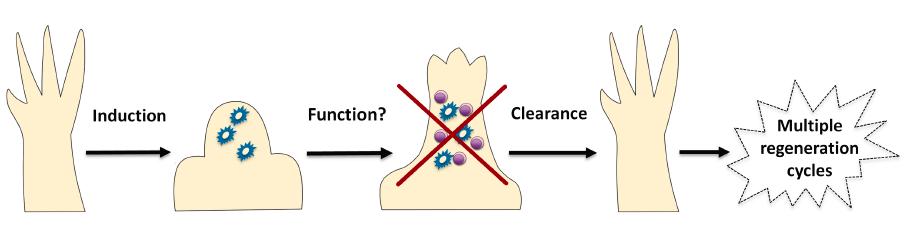
To tackle this problem, the first requirement was to find a way of identifying senescent cells within salamander cells and tissues. Luckily, at that point we had already embarked on a project to investigate the roles of the tumour-suppressor p53 in regeneration5. With my DNA repair background, I couldn’t overlook the fact that p53 is a well known inducer of cellular senescence upon DNA insults and hence took advantage of this to find ways of inducing and detecting cell senescence in salamander cells, such as the well-established senescence-associated beta galactosidase staining. This allowed us to probe some interesting questions, starting with whether senescence occurs at all in salamanders. The short answer is yes, though very few senescent cells are present in normal tissues. In contrast –and quite unexpectedly- we found that they are strongly induced during limb regeneration. But, by the time the limb had regenerated, those senescent cells were gone. Interestingly, we did not detect any senescence induction during normal limb development, indicating that this phenomenon of induction and disappearance is specific to regeneration.
Given the high percentage of senescent cells induced in each round of regeneration, an initial hypothesis was that repetitive amputations should lead to an increase in the number of senescent cells in the regenerating structure. However, as foreshadowed above, this was not the case, with no senescent cells remaining even after five rounds of regeneration. In addition, and even more surprisingly, we found no age-related increase in the percentage of senescent cells in normal salamander tissues, in contrast to observations in most other organisms. This suggested that effective mechanisms of senescent cell clearance operate in salamanders, in normal and regenerating tissues. To test this further, we took advantage of the suitability of the salamander system for implantation experiments, and found that implanted senescent cells were selectively, efficiently and rapidly cleared from normal and regenerating tissues. Hence, the obvious next step was to characterise the clearance mechanism.
At the time, evidence was starting to emerge supporting a role for the immune system in eliminating senescent cells in certain pathological contexts. Therefore, we decided to probe whether the immune system, and in particular the macrophage, was part of the clearance mechanism. Using the well-established DiI and clodronate salt liposome system for labelling and depleting macrophages in vivo, we demonstrated that these immune cells are readily recruited to sites of either endogenous or implanted senescent cells, and that specific elimination of macrophages prevents senescent cell clearance within salamander tissues. Hence, this revealed that the macrophage is an essential piece of this effective immunosurveillance machinery. Ongoing studies are directed at characterising this mechanism further, both trying to determine its cellular components as well as the signals that mediate immune-senescent cell interactions – with a focus on the differences between salamanders and mammals. Clearly, unravelling this phenomenon will be important both from a basic science as well as a therapeutic perspective, as senescent cells have recently become therapeutic targets for the control of age-related diseases. Hence, salamanders could inspire strategies aimed at the efficient targeting of senescent cells for the promotion of healthy Ageing.
Overall, our work revealed that senescent cells are recurrently induced during regeneration of complex structures in salamanders and are subject to a strict mechanism of macrophage-mediated clearance (Figure 1). From a wider perspective, our findings have two important implications. First, that highly efficient mechanisms to eliminate senescent cells throughout lifespan do operate in certain species, and these –as in the case of salamanders- constitute interesting models for the study of senescence regulation and immunosurveillance. Second, that the transient induction of cellular senescence may play a positive role in regenerative processes, a provocative concept that is yet to be addressed.
Figure 1. Senescent cells are induced (by as yet unidentified stimuli) during salamander regeneration, where they may contribute to various aspects of this process. As regeneration proceeds to more advanced stages, senescent cells are cleared by an efficient mechanism of macrophage dependent immunosurveillance, which results in a regenerated limb devoid of senescent cells. This allows for the possibility of multiple rounds of regeneration through lifespan as well as avoiding the disadvantages of senescent cell accumulation.
Our observation that senescent cells are recurrently induced in regeneration was initially surprising, as cellular senescence is traditionally understood as a process with negative outcomes at the organism level. Indeed, from an evolutionary perspective, the persistence of cellular senescence as a genome safeguarding mechanism has been questioned many times: why would a damaged cell undergo senescence, whereby cells remain within the tissues and secrete factors that ultimately lead to their disruption, when it could simply be eliminated through apoptosis? Is it simply that its negative effects are felt during the post-reproductive period, and hence not selected, or is there another explanation? Emerging evidence supports the latter, as a number of studies suggest that senescent cells play positive roles in certain contexts. For example, recent reports support a role for cellular senescence in tissue remodelling during mouse development6,7, as well as in wound closure8. Hence, it is possible that the senescent cells that are induced during regeneration could play important functions in this process – a hypothesis that I am addressing further. This research will not only shine new light into the process of regeneration, but into our understanding of the function, regulation and raison d’être of cellular senescence.
Max H Yun, PhD
Institute of Structural and Molecular Biology, University College London
LTRR-London Tissue Repair and Regeneration Meeting Series: www.ltrr.co.uk
References
1. Eguchi, G., Eguchi, Y., Nakamura, K., Yadav, M., Millán, J., & Tsonis, P. (2011). Regenerative capacity in newts is not altered by repeated regeneration and ageing Nature Communications, 2 DOI: 10.1038/ncomms1389
2. Yun, M., Davaapil, H., & Brockes, J. (2015). Recurrent turnover of senescent cells during regeneration of a complex structure eLife, 4 DOI: 10.7554/eLife.05505
3. van Deursen, J. (2014). The role of senescent cells in ageing Nature, 509 (7501), 439-446 DOI: 10.1038/nature13193
4. Sousa-Victor, P., Gutarra, S., García-Prat, L., Rodriguez-Ubreva, J., Ortet, L., Ruiz-Bonilla, V., Jardí, M., Ballestar, E., González, S., Serrano, A., Perdiguero, E., & Muñoz-Cánoves, P. (2014). Geriatric muscle stem cells switch reversible quiescence into senescence Nature, 506 (7488), 316-321 DOI: 10.1038/nature13013
5. Yun, M., Gates, P., & Brockes, J. (2013). Regulation of p53 is critical for vertebrate limb regeneration Proceedings of the National Academy of Sciences, 110 (43), 17392-17397 DOI: 10.1073/pnas.1310519110
6. Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, & Serrano M (2013). Programmed cell senescence during mammalian embryonic development. Cell, 155 (5), 1104-1118 PMID: 24238962
7. Storer, M., Mas, A., Robert-Moreno, A., Pecoraro, M., Ortells, M., Di Giacomo, V., Yosef, R., Pilpel, N., Krizhanovsky, V., Sharpe, J., & Keyes, W. (2013). Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning Cell, 155 (5), 1119-1130 DOI: 10.1016/j.cell.2013.10.041
8. Demaria, M., Ohtani, N., Youssef, S., Rodier, F., Toussaint, W., Mitchell, J., Laberge, R., Vijg, J., Van Steeg, H., Dollé, M., Hoeijmakers, J., de Bruin, A., Hara, E., & Campisi, J. (2014). An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA Developmental Cell, 31 (6), 722-733 DOI: 10.1016/j.devcel.2014.11.012


 (7 votes)
(7 votes)
I don’t understand why the appearance of senescent cells in regenerating limb suggests that they positively contribute to the regeneration process.
Doesn’t the fact that clearance of senescent cells improve regeneration support the opposite conclusion?
Dear Manuel, thank you for your comment. The reason for stressing this possibility is that the timing and distribution of senescent cells in the regenerate suggest that these cells could be actively induced during the process (rather than being just a result of the initial tissue injury), and the fact that these senescent cells secrete a variety of compounds as part of their senescent phenotype (such as growth factors, MMPs, TIMPS and chemokines) that affect their microenvironment and could promote tissue remodelling. Indeed, transient positive functions for senescent cells have been reported during mouse development. However, these cells need to be eventually cleared, as their persistence leads to tissue disruption and malfunction – for example, their secretory phenotype can lead to permanent inflammation and ‘constant’ tissue remodelling. Basically, the evidence that is gradually accumulating suggests that senescent cells can act as a double-edged sword: they can have positive functions, when transient, but lead to negative effects in tissues, when permanent. This is why, in light of our observations during regeneration of complex structures, I suggest that the senescent cells that appear during regeneration of complex structures could play a positive role, but then they need to be eliminated by this highly efficient mechanism of immunesurveillance so they do not exert any negative effects in the new structure or future regeneration rounds. Yet, whether they contribute to the process is still in the realm of the hypothetical – I hope I will be able to tell you more on this soon, as it is an issue that I am currently addressing.
Hi,
Regarding the regeneration (took place in your experiment) it would not be really part or due to the previous senescence stage. Senescence and senescent cells are not able to induce regeneration of the skin fibroblasts in the aged person who will be keeping the wrinkles until death.