A day in the life of a mayfly lab
Posted by isabelalmudi, on 5 January 2017
I am Isabel Almudi, a postdoctoral researcher in Fernando Casares’ lab, at the Andalusian Centre for Developmental Biology (CABD) in Seville, Spain. In the lab we are focused on studying the control of organ size and identity during development and evolution.
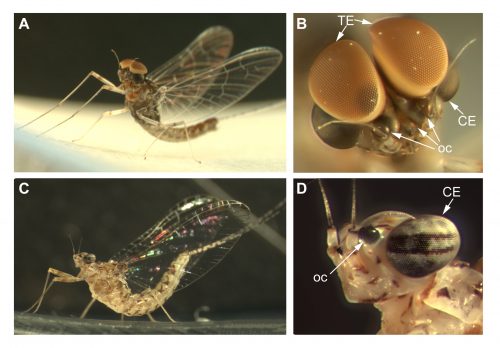
The lab uses the development of insect eyes to investigate the mechanisms and gene networks that regulate tissue growth and final size. Most of our projects use Drosophila melanogaster as a model, but we have recently established two other systems, Episyrphus balteatus (the marmalade hoverfly) and Cloeon dipterum (a mayfly) to address how these mechanisms have changed during Evolution to give rise to the huge diversity found in insect visual systems.
Cloeon dipterum belongs to the Baetidae family of Ephemeroptera, which together with dragonflies and damselflies (Odonata) form the ancient group Paleoptera. Fossil records date ephemeropterans to the Carboniferous, being these insects the first ones that developed wings. This order has a hemimetabolous development, comprising three different phases: nymph, sub-imago and imago. The Baetidae family of mayflies, including C. dipterum, exhibits a striking sexual dimorphism, consisting in the presence of an extra pair of dorsal eyes in males. Females of this species harbour two different types of eyes: the lateral compound eyes and the three frontal ocelli, typical of insects whereas males also develop a pair of extraordinary enlarged compound eyes called “turbanate” due to their shape (Figure 1).
The mayflies have a life cycle that is divided in two main phases, an aquatic phase and a terrestrial phase. The nymphs are aquatic and they can live for several months in freshwater streams, undergoing several moults until they reach their final size. Then, they emerge from the water as sexually immature subimagos that have to moult once more to acquire their sexual maturity. Thousands of adult individuals form swarms and mate while flying some metres above the water. Finally, the females lay the fertilised eggs onto the surface of the water, where they will develop and later hatch as nymphs. One of the particularities of C. dipterum is that it is one of the few ovoviviparous Ephemeroptera species. Thus, once the adult female lays the eggs, they immediately hatch as tinny swimming nymphs (Figure 2).

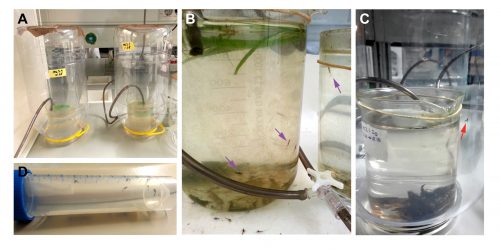
Closing the life cycle in the lab was one of the first challenges we encountered. Due to space limitation and lack of infrastructure (we do not have a pond inside the institute to leave a swarm of mayflies mate freely), we have to perform forced copulation with them [1]. We grasp the female by the wings with a pair of forceps and place it in an inverted position. The male is placed against the female allowing direct contact between the external genitalia of both. The male genitalia forceps clasp around the female abdomen and the copula starts. The duration of the matings can vary from few seconds to several minutes (Figure 3). After the mating, we keep the females in a petri dish with some wet paper and wait for 15 days, until the embryos have fully developed inside the female abdomen. It is from this moment that we put the females onto the surface of water to allow them to deliver their offspring. We keep the nymphs in beaker glasses with water and air bubbling. These beakers are inside PET bottles to prevent the subimagos to fly away when they emerge from the water (Figure 4).

A typical day in a mayfly lab starts collecting the subimagos and imagos that have emerged and moulted during the night. These will be the specimens that we will use two days later for our crosses, so we put them in a falcon tube with some wet paper to maintain the humidity. As adults, mayflies do not feed, they lack mouth parts, so we do not need to worry about providing them with some food, we just need to avoid their desiccation. Nymphs, on the contrary, are very voracious. We need to feed them every morning with algae or vegetarian fish food flakes. It is very convenient to be in an institute with several zebrafish and medaka labs, as they are our main algae providers, just by scratching the algae that grow on the surface of the fish tanks. After taking care of the nymphs we can start performing some experiments. Having the culture established in the laboratory permits us to select the nymphs at the appropriate developmental stage to study the ontogeny of the turbanate eye and the genes that are involved in the process.
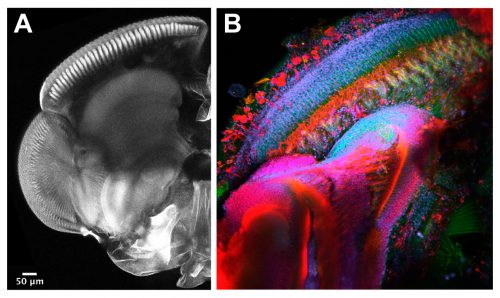
The techniques we use are not very different from the ones applied in other Evo-Devo and Developmental Biology labs working with well-established model organisms. Therefore, widespread procedures like imaging and gene expression studies are performed on a daily basis with the mayflies. Confocal microscopy and immunostainings are used to study the morphology of the turbanate retina and its associate brain centres along its development. Markers, such as antibodies for proliferation or specific cell types, serve us to investigate growth rates and patterning dynamics of the new organ (Figure 5). We have also generated some tools to investigate gene expression. We are able to search for genes in a transcriptome that we have produced and to look for their spatial expression using in situ hybridization at the desired developmental points. Our next goal is to establish functional tools in order to test the candidate genes we are identifying from our gene expression experiments.
Setting up a new model in the lab presents some challenges, but it will help us to answer many questions regarding the origin and evolution of traits that appeared for the first time in insects, thus, every goal we accomplish is really rewarding and exciting.
References and Notes
- McCafferty, W.P., and Huff, B.L., Jr. (1974). Parthenogenesis in the mayfly Stenonema fermoratum (Say) Ephemeroptera: Heptageniidae). Entomological news 85, 76-80.
- Isabel Almudi holds a MSCA IEF 657732 fellowship funded by the H2020 program of the European Commission.
-

The Mayfly team. Carlos Martin-Blanco (master student, left) and myself.


 (18 votes)
(18 votes)
Dear Nikki,
Males of C. dipterum start developing their turbanate eyes during nymphal stages, so as soon as they are visible (around 4mm nymphs) you can tell them apart very easily. I am not sure about other species without this sexual dimorphism, I guess you can always open them and check the gonads. We are developing some genomic tools now, so hopefully we will obtain sex specific signatures in order to genotype the nymphs in the next future.
I wanted to know how you created the nymphs in the laboratory, do you have any literature to recommend?