In Development this week (Vol. 143, Issue 1)
Posted by Seema Grewal, on 5 January 2016
Here are the highlights from the current issue of Development:
Making and shaping the lung epithelium

Gas exchange in the lung occurs across the alveolar epithelium, which consists of flattened AT1 cells that comprise the gas exchange surface and cuboidal surfactant-producing AT2 cells. Both cell types are generated from a bipotential progenitor, but the events surrounding cell differentiation and morphogenesis of the alveolar structure are still poorly understood. On p. 54, Jichao Chen and colleagues investigate the differentiation, morphogenesis and plasticity of mouse AT1 cells in the peri- and postnatal lung. They find that, although alveolar surface area increases dramatically in the weeks after birth, AT1 cells do not appear to proliferate; increase in surface area is achieved by a ∼10-fold increase in cell size. AT1 cell differentiation involves a two-step process of cell flattening and cell folding as alveolar septation occurs. Moreover, signals from the AT1 cells may regulate alveolar angiogenesis and secondary septation. Finally, although AT1 cells are highly morphologically differentiated, they still show some degree of plasticity: overexpression of SOX2, which promotes airway differentiation, in developing or mature AT1 cells causes retraction of the cellular extensions and induces proliferation. Together, these data shed light on the mechanisms underlying postnatal lung development and add to accumulating evidence for an unexpected degree of plasticity in the lung epithelium.
The evo-devo of neural progenitors

Underlying the intricate complexity of the vertebrate brain is a complicated set of developmental programs regulating proliferation and differentiation of the different regions and neuronal types. In the mammalian neocortex, two major types of progenitor cells have been characterised: apical progenitors (APs) that divide at the apical surface of the ventricular zone and basal progenitors (BPs) that divide in the subventricular zone. BPs can be further subdivided into different types, including intermediate progenitors expressing the Tbr2 marker and cells with stem cell-like properties: basal radial glial cells (bRGs). To date, bRGs have only been characterised in mammals, but the evolutionary origin of different BP populations is uncertain. Now, Tadashi Nomura and co-workers (p. 66) characterise a bRG-like population in the chicken pallium (a region of which is homologous to the mammalian neocortex). These cells share many properties with mammalian bRGs, including their morphology, position, orientation of mitoses and response to various genetic manipulations. The authors further show that this lineage is distinct from Tbr2+ progenitors, which in the chick – unlike in the mouse – appear to be non-proliferative. Furthermore, surveying a range of amniotes and amphibians suggests that BPs are quite widely distributed in vertebrates, suggesting they may be a more ancient evolutionary innovation than previously thought.
A balancing act at the synapse

Matrix metalloproteinases (Mmps) and their inhibitors (Timps) are thought to be important for synaptogenesis, but their roles are poorly understood – at least in part because there is significant complexity and redundancy in the mammalian matrix metalloproteome. Kendal Broadie and colleagues (p. 75) have therefore turned to Drosophila, which have just two Mmps and a single Timp, as a simpler system to assess the roles of Mmps and Timps at the developing neuromuscular junction (NMJ). They find that depletion of either mmp1 or mmp2 alone leads to increased synaptic architectural complexity as well as elevated functional neurotransmission. Surprisingly, however, simultaneous loss of both Mmps or overexpression of Timp, has a much weaker phenotype. It appears to be the balance of Mmp1 and Mmp2 on both pre- and postsynaptic sides of the NMJ that is critical for appropriate synapse formation. The authors find no ultrastructural defects, but rather that dysregulation of Mmp activity impacts synaptic Wnt signalling, with the level and localisation of the Wnt co-receptor Dlp impaired in Mmp mutants. Although the precise roles of and the interplay between Mmp1, Mmp2 and Timp have yet to be fully understood, this system provides a powerful new model for investigating the roles of the matrix metalloproteome during synaptogenesis.
Pausing on the way to pluripotency

The generation of induced pluripotent stem cells (iPSCs) has revolutionised the stem cell field, opening up avenues for both basic and translational research. However, there is still much to understand about the mechanisms underlying reprogramming to the iPSC state, particularly in human. On p. 15, Takashi Tada and colleagues report the isolation of stable ‘intermediately reprogrammed stem cells’ (iRSCs) that are paused in their progression to pluripotency. These cells, generated by transient expression of the reprogramming factors Oct4, Klf4, Sox2 and c-Myc, express some pluripotency markers, such as endogenous SOX2 and NANOG, but have not yet undergone mesenchymal-to-epithelial transition (MET) or upregulated endogenous OCT4. The iRSC lines are stable over multiple generations, but can easily and efficiently be induced to continue reprogramming to an iPSC-like state by culture at high density. The authors use these iRSC lines to characterise the order of events during reprogramming, finding that in human, unlike in mice, induction of endogenous OCT4 expression precedes MET. Importantly, however, this expression is initially unstable, and some cells revert to an OCT4− state and show signs of lineage commitment. These cells lines should provide a valuable tool for further investigation of the mechanisms underlying reprogramming to pluripotency of human cells.
PLUS…
Future developments: your thoughts and our plans
![]() As a journal with its community very much at its heart, we here at Development believe it is essential to ensure we take your opinions into account when planning for the future. It was with this ethos in mind that we recently carried out a community survey looking at how well the journal reflects the current state and future directions of the field. Here, we discuss the results of this survey and the future directions of the journal. See the Editorial on p. 1
As a journal with its community very much at its heart, we here at Development believe it is essential to ensure we take your opinions into account when planning for the future. It was with this ethos in mind that we recently carried out a community survey looking at how well the journal reflects the current state and future directions of the field. Here, we discuss the results of this survey and the future directions of the journal. See the Editorial on p. 1
When stem cells grow old: phenotypes and mechanisms of stem cell aging
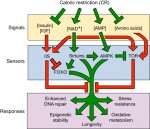 All multicellular organisms undergo a decline in tissue and organ function as they age. Here, Michael Schultz and David Sinclair discuss recent advances in our understanding of why adult stem cells age and how this aging impacts diseases, lifespan and potential therapies. See the Review on p. 3
All multicellular organisms undergo a decline in tissue and organ function as they age. Here, Michael Schultz and David Sinclair discuss recent advances in our understanding of why adult stem cells age and how this aging impacts diseases, lifespan and potential therapies. See the Review on p. 3
Featured movie
Our latest featured movie shows cardiac contractility and circulation near the heart of a zebrafish and is from a recent paper by Torres-Vázquez and colleagues. They performed a genetic screen in zebrafish and identified reck as a key modulator of Wnt signalling, required in the brain endothelium for intra-cerebral vascularisation and proper expression of barriergenesis markers. Read their paper: http://bit.ly/1PaUYHi


 (No Ratings Yet)
(No Ratings Yet)