Discovery Through Collaboration: Brain Lymphatic Endothelial Cells
Posted by Shibata-Germanos, on 8 August 2017
Looking back on the journey: Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development eLife van Lessen et al., 2017
Just over two years ago, while I was a Masters of Neuroscience student at University College London, I became interested in the emerging concepts of brain lymphatics and sleep dependent macromolecule clearance within the brain. Until quite recently, the brain was considered to be without lymphatic vasculature or dedicated mechanisms of clearance of its own. Then in 2013, Lulu Xie of the Nedergaard lab published a blockbuster paper in Science that suggested that sleep facilitates fluid and metabolite clearance from the brain (Xie et al., 2013). Following in 2015, a double header in Nature and The Journal of Experimental Medicine (Louveau et. al. 2015, Aspenlund et al. 2015) demonstrated that lymphatic vessels exist within the dura mater of mouse and human meninges. Together these papers opened an entirely new outlook for how our energy intensive brain might maintain homeostasis, with potential implications for neuropathology and degenerative disease. I was attracted to the interdisciplinary aspects of this budding field, and the topic became a fundamental obsession for me after several involved discussions with Dr. Jeff Illif who had written the original brain paravascular clearance paper (Illif et al., 2012) and moved from the Nedergaard lab to head his own at Oregon Health and Science University in 2013. I was deeply encouraged by the time he took to act as a sounding board and to offer mentorship and direction as I decided how to pursue this field for my PhD.
I had a potential idea for how to tackle this field from a new angle after Dr. Jason Rihel from UCL’s Department of Cell and Developmental Biology gave a lecture for the Master’s program, during which I discovered that his lab uses molecular and genetic techniques to study sleep using zebrafish. I found his approach and style as a creative, big-picture scientist appealing. Zebrafish also made sense to me as a model through which to investigate this complex clearance mechanism, as they are small and optically transparent throughout larval stages, allowing for accessible imaging and high throughput studies. However, when I approached Dr. Rihel (Rihel et al. 2010) with a PhD proposal to investigate these processes in zebrafish, he warned me that not only would this be a new area for his lab, requiring new methods, but we also might find that zebrafish simply lack brain lymphatics/clearance. To finally convince Dr. Rihel that it was worth investigating, I poured over these new papers along with zebrafish papers describing lymphatic development, many of which came from a well-known lymph and vascular lab in Muenster, Germany led by the seasoned scientist, Professor Stefan Schulte-Merker.
About the same time, in Professor Schulte-Merker’s lab, a postdoctoral researcher by the name of Max van Lessen had also caught the brain lymphatics ‘bug’. Digging through the same literature, Max had his own “eureka” moment when he realized that the transgenic vascular and lymphatic lines, including a few created by his colleague Andreas van Impel, might have labelled the brain. Such transgenic tools would allow for dynamic investigation of the origins and molecular properties of brain lymphatics, if they were present and conserved in bony fish.
Serendipitous timing brought Professor Schulte-Merker to our doorstep last March for an unrelated meeting with our colleague and neighbour, Professor Steve Wilson. When I heard from my colleague Dr. Eirinn Mackay that he was down the hall, I jumped up and caught him as he was heading out the door. Taking advantage of the brief moment, I thrust my hand at him for a hearty shake and I began excitedly outlining that I believed brain lymphatics found in mice and humans would be conserved in zebrafish. I told him that in some of the images from papers, I had seen evidence of lymphatic markers present in the head of the zebrafish and wanted to know his opinion. Professor Schulte-Merker smiled and nodded. “Indeed, we have noted this and are looking into it.“ I asked out of curiosity why had everyone in the zebrafish vascular community focused exclusively on the trunk of the fish? Most images of zebrafish lymphatics I found in the literature had frustratingly cropped the top of the head from full view. Professor Schulte-Merker smiled wryly and stated that “everyone was so engrossed with the peripheral system in the past that no one ever bothered to look up.” It struck me that so much of discovery is about simply “looking up”. Not being afraid to challenge long standing norms and established or popular avenues of thought to follow instincts that bring us to look with new eyes upon something we have seen before a billion times.
I also discovered that science is, more often than not, luck, happenstance, and the willingness to cooperate to get more done. We can work alone and hoard, or we can branch out and be, as my friend Seanne in California always likes to put it, “better together”. My PhD supervisor Dr. Rihel cultivates an atmosphere of collaborative research, and his fearlessness in sharing ideas and taking chances paved the way. The Schulte-Merker lab didn’t hesitate to reciprocate in the same spirit, and immediately a cooperation was forged. To begin parallel investigations into brain lymphatics, the Schulte-Merker lab sent us a combined transgenic line with kdr-l expression labelling all vasculature in mCherry (Hogan et al., 2009) and flt4 (Vegfr3), an established venous-lymphatic marker in mCitrine (van Impel et al., 2014).
We at the Rihel Lab have an interest in the conservation of these systems and mechanisms to ultimately elucidate the impact on potential clearance through sleep, and the Schulte-Merker lab thinks about this problem through the angle of vascular and lymphatic origin and development. We have complementary skill sets that allowed the work to begin at an incredible pace, building a whirlwind storyline. My colleagues often remark that this project made them feel like they had been strapped to a rocket ship.
The Mystery Cells
To help with the anatomical search for brain lymphatics, I recruited my remarkably talented second supervisor as a co-investigator (Dr. Tom Hawkins), as he is one of the most highly inquisitive people I have met, with a wide breadth and depth of technical expertise which would clearly be needed. We began by looking at adult transgenic brains, carefully dissecting and removing them using confocal microscopy to examine both the surface of the brain as well as the interior portions of the skull. What we saw initially was somewhat astonishing. We had imagined we might find lumenized vessels; however, along much of the surface of the brain were what looked to be flt4 positive cells of some sort, glowing brightly in mCitrine, clearly differentiated from the kdr-l blood vascular marker in brilliant mcherry. While kdr-l expression labels both arteries and veins, flt4 expression is an established marker of venous and and lympahtic endothelium. Importantly, our cells were entirely negative of kdr-l expression, which in the trunk can only be found in lymphatic vessels.
They possessed large inclusions and spindly processes which appeared to be connecting each to the other in close proximity to surface vasculature. A Sir Henry Welcome Fellow in our lab, Sabine Reichert, took one look at the images and without blinking stated, “they look like some sort of endothelial cell type.” It quickly became clear that she was right.
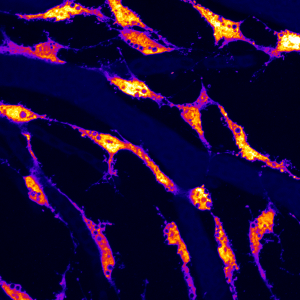
We dove in with immunohistochemistry using anti GFP and RFP antibodies followed by cryosections in adult brains to investigate carefully where these cells were positioned. Our cryosections revealed that these cells populated the meninges surrounding the entire brain exclusively and were associated with particular vessels diving into the parenchyma from the meningeal layer. This was an exciting moment for us as we recognized that the work done in mice and in humans had placed brain lymphatics in the meninges and our cells were positioned in a similar location. Although much work remained, we in London felt we were on track and may have identified a brain lymphatic system in the fish, suggesting evolutionary conservation within the vertebrate lineage.
Origins
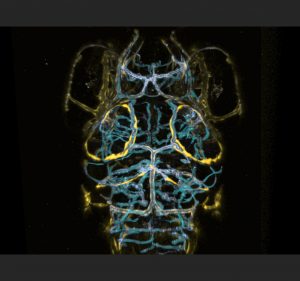
Around the same time Professor Schulte-Merker and I had met, Max and the Schulte-Merker lab in Germany had found beautiful evidence of head lymphatics in the embryonic brain. Max had been capturing live images from the earliest stages of development onwards, patiently waiting to see when, how, and from where expression of these flt4 positive structures would emerge to get a hint of their developmental origin. They reasoned that if peripheral lymphatics sprout from venous structures early in development, perhaps the same would occur in the brain. While training the confocal microscope on the choroidal vascular plexus (CVP) and mesencephalic vein (MsV) which loops around the optic tectum of the zebrafish brain, they saw a clear sprout comprised of flt4 positive cells emerging around 56 hours post fertilization (hpf) from the CVP that then grew in an arc along the MsV at the edges of the tectum in parallel to the vasculature. Residual kdr-l expression in these flt4 positive cells suggested differentiation from venous endothelium.
Over the course of three days, this flt4 positive ring from what we began calling the stereotypical tectal “loop”, a cohort/string of loosely connected single cells which aligns closely to the MsV. In the peripheral nervous system of zebrafish, angiogenesis begins with the sprouting and development of arteries, followed by the concurrent development of veins and lymphatic vessels with both requiring three key genes: ccbe1, vegfc and flt4. Both lymphatic sprouts and venous sprouts initially originate from the posterior cardinal vein and ultimately form their own independent structures as they grow towards the myoseptum (van Impel et al., 2014). Since these novel brain cells sprouted from a vein and expressed flt4, Professor Schulte-Merker remarked that they were most likely lymphatic in origin.
Macromolecule Uptake
If these novel cells were in fact functionally similar to mammalian lymphatic structures, Max hypothesised that these cells may have function in the uptake and clearance of macromolecules, similar to what has been reported for murine brain lympahtic vessels. Incredibly, tracers injected either into the tectal neuropil or into the hindbrain ventricle accumulated at flt4 positive cells forming the tectal loop within minutes of injection. This uptake is so rapid and specific that when I first showed Dr. Rihel a similar dye injection experiments that my colleague Dr. Chintan Trivedi and I had performed on the two-photon microscope in a wild type fish, he thought I was showing him an image of the lymph transgenic line, as the dye took on the precise location and shape of the flt4 positive cells! With so much activity going on in both of our labs, we realized that it was time to have an in-person meeting to facilitate discussion and avoid excessive experimental overlap.
I made a pre-Christmas journey to Muenster to mutually share our findings along with new ideas and techniques. During my time there, I was also introduced to Andreas van Impel, another postdoctoral researcher in the Schulte-Merker lab, with a humble and steadfast demeanour who had created a number of the stunning complex lymphatic lines we were currently using. He brought a great deal of experience and insight to our discussions and grounded us with his thoughtful interjections. When Max presented his work, we both were so excited that we were wildly gesticulating and scribbling notes. At one point, one of us climbed atop of the table to point out a key piece of research from an old paper from the 1930’s, with Andreas calmly leaning in with an amused smile on his face. It was one of the most vibrant scientific visits I have had, and it solidified a strong sense of teamwork. Face to face contact I found is the cornerstone of collaboration. Trust must be earned and built, while looking one another in the eye, so colleagues can both sense and see our intentions, and know we are all labouring for the same goal.
Shortly after my visit, Max shared he had mused that the cells had macrophage or dendritic-like characteristics, even though their lymphatic origin suggested a novel cell type. This had Max hunting after shared receptors or other common characteristics, which may offer further clues about our cells. In this reading, Max discovered a universal pattern recognition receptor called the mannose receptor found in both macrophages and dendritic cells and was a major way in which cargo was endocytosed into these cell types. Thus, he decided to apply both Pyrimidyn-7, a quintessential blocker of dynamin-dependent endocytosis, and mannan, a bacterial polysaccharide that binds to and saturates the mannose receptor in independent experiments to see if he could stop tracer uptake. He shared on a call that he was left deeply puzzled as it looked like the tracers had still localized to the flt4 positive cells anyway, even in the presence of the endocytic blocker.
Fortunately, Max had also injected the pH sensitive dye (pHrodo-advin) which would only fluoresce when it encountered an acidic environment inside of a cell. In previous experiments, he had seen both the tracers and the pHrodo fluoresce at the flt4 positive cells together, demonstrating for the first time lymphatic uptake of macromolecules at the single cell level. Being in the habit of injecting pHrodo along with his usual tracer, he also used this dye cocktail in his search for an effective concentration of mannan or Pyrimidyn-7, which revealed that the usual fluorescent tracer still accumulated at the cells. However the injection of either blocker effectively stopped the internalization of pHrodo. It didn’t make sense! How could the fluorescent tracer be taken up, but the pHrodo not? Suspicious, he took a closer look, and realized that the usual fluorescent tracer had merely localized to the membrane of the cell and was not actually being internalized. 3D reconstructions revealed that the fluorescent tracers were lodged into the membrane. If pHrodo was injected before the blocker it still happily fluoresced in the acidic lysosomal compartments within the cell but never co-localized with the fluorescent tracers lodged in the membrane after endocytic blockage.
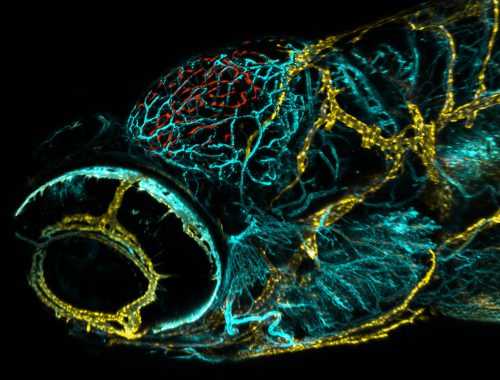
Thus, it seemed that one process “docks” the tracer to the cell membrane while subsequent cargo internalization into acidic compartment is likely mannose receptor dependent. If it hadn’t been for his habit of using the pHrodo he may just have given up thinking the blockers hadn’t worked. I learned a few valuable lessons from Max’s results. First, I learned that experiments don’t have to be complicated and showy. They can often be incredibly straightforward. The other lesson was to always look closer and deeper before walking away from an experiment in frustration. So much of science emerges from happy accidents. We hear it again and again, and that is because it’s just so true.
Max and his colleagues expanded the analysis to additional lymph markers as well as markers for macrophages, etc. For example, they examined transgenic lines labelling classic lymphatic markers such as Lyve1 (Okuda et al., 2012) and Prox1 (van Impel et al., 2014) to further confirm these cell’s lymphatic identity. Much to our combined excitement, each lymphatic line labelled the quintessential tectal loop. Finally, to rule out that these flt4, prox1, lyve1 positive cells were macrophages, Professor Schulte-Merker suggested breeding the lyve1 line in DsRed (Okuda et al., 2012) with the mpeg1 macrophage line in GFP (Ellett et al., 2011). This showed clear differences between the cell types based on location, morphology, and expression. To completely eliminate the possibility, they also examined whether the morpholino knockdown of the macrophage transcription factor pu.1 had any effect on the lymphatic tectal loop. Andreas injected morpholinos, and after a tense few days of waiting for the results, the experiment revealed that our flt4 positive cells remained undisturbed, even as all macrophages were eliminated. We were elated, and we all finally allowed ourselves to believe that we had a novel cell-type on our hands.
Back in London, Tom and I began our hunt for the identity of these cells from a very different angle, using electron microscopy to obtain a definitive view of the location and ultrastructural features of these cells in relation to blood vasculature. Tom has had decades of experience with transmission EM, so he warned me not to get too excited, as disappointment was likely. Using the enormous TEM microscope with the assistance of Mark Turmaine: the quiet EM master, we happily navigated our way over the ultrathin slices in what, to me, was reminiscent of the broadcasts from the first moon landing. To our delight, and despite the forewarning, our first EM run was crystal clear with excellent structural resolution. The cellular landscape was wild and extraordinary, full of intricate interdigitations of membranes and strangeness all around. Guided by venerable EM studies on goldfish meninges, we could clearly identify a distinct meningeal tri-layer, inside which the cells that contained these large inclusions were exclusively found. We were able to immediately identify structures with multiple large inclusions in close proximity to surface brain vasculature with the size and distribution shown in our confocal images. They were clearly separable from perycites, vascular endothelial cells, and their basement membranes.


We combed over mammalian and teleost EM images of the meninges to try to identify these cells. One candidate was the Fluorescent Granular Perithelial Cells (FGP), which were discovered and characterized in rodents by Maso Mato in the 1980’s. Mato cells were known to reside in the meninges and contained numerous, heterogeneous inclusions (Mato et al., 1986, 2002), and we had several hours of heated debates in Dr. Rihel’s office as we stared at side-by-side comparisons of classic EM images and our new data. Ultimately, we decided that our cells were most probably distinct from Mato cells, or at least they represented a specific subtype, as our new cells’ inclusions were less heterogeneous than expected for Mato cells, and they had morphologically distinct shapes.
The ”Competition”
As the first part of our story of these cells was beginning to wrap up, Max and I were made aware that two other labs had come to similar conclusions to us. Suddenly, we were plunged into a race in which we weren’t precisely clear who had what, and when, in entirety.
Moments like these, when you find out other labs are working on similar avenues, can be stressful at best, and to be frank, Max and I had moments of panic together. The prospect of potentially being “scooped” looms heavy above all of our heads at any given moment. Each one of us is deeply connected to our research and have spent countless hours sweating and toiling over our projects. They become a part of us. And hearing that others have found something similar can be particularly disorienting as discovery, though made for the common good, can feel so deeply personal. Professor Schulte-Merker and Dr. Rihel kept insisting that each lab would be contributing to the scientific enterprise and bring novel insights into the nature of these cells.
This also meant that the community would be excited about this discovery. In retrospect, with the three respective papers now published, (Galanternik et al., 2017, Bower et al., 2017, van Lessen et al., 2017), it is clear that each lab approached these cells from unique perspectives. Galanternik et al. discovered these cells using a transgenic mannose receptor line and indicated that they believed these to be fluorescent granular peritheliaI cells (FGP or Mato Cells) (Galanternik et al., 2017). Bower et al. utilized the transgenic lyve1 lymphatic line to reach their discovery, and amongst other interesting findings they ablated these cells to find that the ablations impacted the integrity and development/regeneration of meningeal vasculature (Bower et al., 2017). Both those papers also performed valuable RNAseq analysis using differential gene expression analysis to rule out the cell’s having blood endothelial, smooth muscle, macrophage, or pericyte identity. This exciting combined evidence opens up an entirely new avenue of research into brain lymphatics in zebrafish.
Arachnocyte, Kumocyte, BLEC
Finally, as we debated what to name these cells, the other labs disclosed the names with which they were identifying the cells in their papers, with Galanternik et. al. calling them FGPs and Bower et al. settling on perivascular Lymphatic Endothelial Cells (pLECs). We ultimately decided on Brain Lymphatic Endothelial Cells (BLECs) to be precisely descriptive of their properties; however, internally we all enjoyed the name we had additionally selected which was Kumocyte. Kumo means spider in Japanese, describing their spidery appearance, they had previously been colloquialised as arachnocytes for the same reason. I think they will always remain Kumocytes to us somehow, as it speaks to their unique morphology and “personality” and we have taken to fondly calling them that within the Rihel lab.
We are grateful for this opportunity to share our experience as we are so often confined by formality, word limits, and jargon that we miss the opportunity to tell “the story”. The story which discusses the more personal aspects of the craft of discovery, especially the journey behind the scenes that keeps all of us all coming back again and again despite the tough days, which are many. After all, in biology and science, we are all just witnesses to nature, merely reporting what we see with awe and wonder. It’s always been there, but there’s that kid in all of us that gets our mind-blown every time we get to be the ones to see something with human eyes for the “first time.“ I also never want to forget on this journey, not just in science, but in life, that we truly are only as good as those we surround ourselves with. Each and every colleague on my floor has contributed, and given their time and expertise without hesitation. They say it takes a village to raise a child…..and so it also goes with raising a PhD student.
We can’t wait to see what this field of brain lymphatics and clearance holds. Certainly, dogma that the brain is without dedicated lymph structures of its own is being rewritten, even in fishes. As we start at the drawing board and re-examine all of our old assumptions, what else will we discover around the corner?
Shannon Shibata-Germanos and Max van Lessen





 (8 votes)
(8 votes)