Don’t eat me!!
Posted by Cecilia Pessoa, on 15 November 2024
When I joined the Zon lab in June 2021, my mentor, Leonard Zon, shared an insightful piece of advice: “A good project always has two questions, one you can answer and one you dream of answering.” In this post, I’ll focus on that dream.
In brief, the question we managed to answer – at least a little bit – is how to instruct the expression of the “eat-me” signal driven by Calreticulin (CALR) and its complementary “don’t-eat-me” signal, driven by beta-2-microglobulin (B2M). In our study (https://www.science.org/doi/10.1126/science.adn1629) , we showed that high levels of reactive oxygen species (ROS) leads to high surface presentation of Calr, which, in turn, leads to high levels of interaction with a macrophage, and clearance of the stressed hematopoietic stem and progenitor cell (HSPC). On the flip side, TLR3 can modulate the expression of CALR together with B2M. Here, the balance between these molecules leads to a scenario where a macrophage interacts with the HSPCs, but does not “eat” them. This intricate signaling impacts clonal diversity, revealing a potential avenue for future immunotherapies targeting mutant or cancerous stem-cell populations while sparing healthy ones.
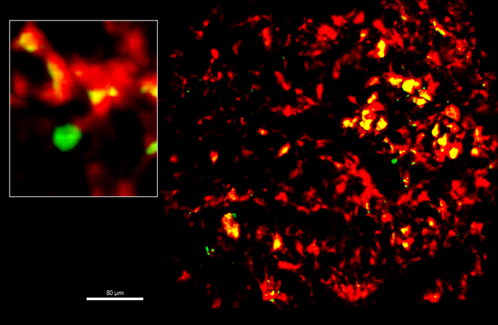
Our uncharted territory lies in harnessing macrophages to selectively target malignant clones. We found higher B2M expression in HSPCs from acute myeloid leukemia (AML) patients with malignant stratification, suggesting that malignant clones may exploit B2M to evade macrophage clearance. This paves the way for drug development aimed at eliminating pre-leukemic and leukemic cells via macrophage-mediated clearance. This idea may also be further explored in aging studies, in which one could teach the macrophage to eliminate aged progenitor cells.
Another aspect of our study that could be further explored relies on a key finding that repetitive elements (RE), including Ltr, are the endogenous ligand of the Tlr3 and triggers b2m expression via the tlr3/irf3 pathway. We observed that these endogenous REs promoted high levels of ISG15, a gene linked to the type I interferon response.
Given the evolutionary conservation of RE and B2M, we explored the significance of this mechanism in both fish and humans, focusing on pathogen infections that are a common threat to both species. Specifically, we examined the role of TLR3 signaling in inducing “emergency granulopoiesis,” a protective process that accelerates neutrophil production during severe infection. Upon poly I:C stimulation, neutrophil populations increased.
Although further studies are needed to strengthen the relevance of this phenomenon, our results suggest that viral stimulation may confer a better fit against opportunistic pathogens by promoting granulocyte differentiation. This observation gets more fascinating if one considers that this increase of type I response could not only alter the emergency granulopoiesis, but also contribute to innate immune training. Seminal studies have shown that type I IFN signaling mediates neutrophil trained innate immunity, mainly in the context of solid cancer. This therefore suggests that RE-triggered type I interferon may play a role in trained immunity—a concept previously explored in cancer but now potentially relevant in other systems.


 (3 votes)
(3 votes)