Electrifying news for embryologists
Posted by Elsa Mazari and Aitana Perea-Gomez, on 17 June 2014
Electroporation: an efficient technique for embryologists
During embryonic development, the specification of different cell types giving rise to the future organs involves a precise spatiotemporal regulation of cell proliferation, migration, and differentiation. Studying these processes requires tools to manipulate gene expression locally in the developing embryo.To this aim, embryologists have widely used the technique of electroporation, consisting in the delivery of exogenous molecules (such as nucleic acids) into targeted cells through electric permeation of the plasma membrane(Calegari et al., 2004; Escoffre et al., 2009; Nakamura and Funahashi, 2012; Swartz et al., 2001). Localised electroporation is achieved by two main approaches depending on the position and the geometry of the targeted tissue (Fig. 1). If the zone of interest surrounds a natural body cavity, one can generate an electric field with large electrodes and inject locally the exogenous molecule (Fig. 1a; Itasaki et al., 1999; Soares et al., 2008). Otherwise, the electroporation solution is homogeneously applied and the electric field is then spatially restricted by, for instance, using needle-shaped electrodes placed in close proximity to the targeted area (Fig. 1b; Davidson et al., 2003; Momose et al., 1999). One important drawback of this second strategy is that electrolysis occurring at the needles surface during pulse application generates harmful chemical species that may result in cell damage and poor embryo survival (Kim et al., 2008; Wang et al., 2010; Wang and Lee, 2013).
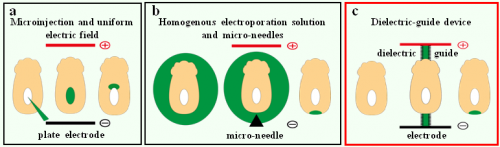
Figure 1. Strategies for localised electroporation in early post-implantation mouse embryos. (a) The first technique involves a uniform electric field and a localized injection of nucleic acids. (b) The second technique relies on a localized electric field and a homogenous nucleic acid solution. (c) Our technique involves dielectric guides where electric field is spatially restricted inside confined channels filled with electroporation buffer.
When embryology meets microfabrication
To circumvent these problems, we developed a system where the electric field generated by large electrodes is conveyed to the targeted zone by narrow channels, also known as dielectric guides, filled with electrolyte (Fig. 1c; Mazari et al., 2014). In this way, efficient electroporation with reduced cell damage is obtained as the electroporated tissue is exposed to a confined electric field while lying far away from the electrode. Moreover, the sample is immobilized by suction in front of the channel, therefore obviating the need for micromanipulation. Interestingly, dielectric guide-based electroporation devices for single cells in culture had been successfully adapted to the on-chip format, with microfabricated electrodes and fluidic channels (Fox et al., 2006; Wang et al., 2010; Wang and Lee, 2013). To design similar tools able to target small embryos or organ explants, the most important parameters are the dimensions of the dielectric guide. Indeed, transfecting as few as 4 or 5 cells of a specific tissue, which approximately represent an average surface of tens square micrometers, requires the guide aperture to be of a similar size. Derived from the microchip industry, microfabrication techniques are particularly well-suited to create microstructures. Through a straightforward and quite simple process, these microengineering techniques then enable the reproducible production of defined shapes and positions at the micrometer scale.
An optimised and collaborative approach
As a proof of principle, we chose to target small cell populations in the visceral endoderm (VE), the outer epithelium of mouse embryos at embryonic day 5.5 of development (E5.5) (Fig. 2a-b). This is a critical time for the establishment of embryonic polarity, with distal VE (DVE) cells undergoing a stereotypical migration that will define the future anterior side (Takaoka and Hamada, 2012). However, existing electroporation procedures for this stage face poor embryo survival and poor reproducibility. First, we conceived an electrical model of E5.5 embryos and used it in numerical simulations to compare the outcome of different electroporation strategies. This analysis demonstrated that the proposed dielectric guides device would permeabilize a more restricted area than current electroporation systems. In addition, we experimentally and systematically investigated pore generation and cell viability to determine the best electrical conditions for efficient electroporation of DVE cells. Constant optimisation by alternative simulations and experimental tests, resulted in the development of dielectric guide-based devices and associated protocols to locally electroporate E5.5 mouse embryos in an efficient, reproducible, and safe way (Fig. 2a-c).
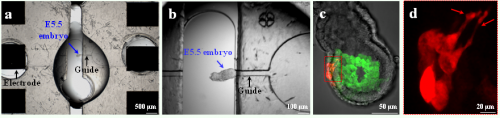
Figure 2. Use of the dielectric guide-based device on an E5.5 embryo. (a) View of the whole system included between the electrodes. An E5.5 embryo (blue arrow) is positioned with its DVE abutting the dielectric guide connected to the cathode, so as to target precisely this tissue. (b) Close-up view of the same embryo. (c) Image of a transgenic Hex-GFP E5.5 embryo electroporated in the DVE with pCAG-mCherry DNA. (d) Close-up view of migrating mCherry expressing cells. The red arrows show cell projections.
We combined our electroporation technique, transgenic mouse lines, and live imaging to study the behavior of VE cells during DVE migration between E5.5 and E6.0 (Fig. 2c). We were able to specifically label subpopulations of follower DVE cells that so far had been difficult to visualize, and to monitor the production of dynamic cellular projections during their migration (Fig. 2d). Furthermore, we demonstrated that our microdevice can be used to electroporate tissues in a wide range of embryonic contexts just by adapting the guides dimensions. A distinctive feature of our work lies in the proposed interdisciplinary approach that brings together expertise from biology to physics. Our strategy coupling numerical simulations, prototype microfabrication, and in vivo testing, provides an optimized and rigorous framework for the design of other tailor-made electroporation devices (Fig. 3). We hope that this work will provide a stimulating example ofthe electrifying interest of microfabrication approaches to developmental biologists.
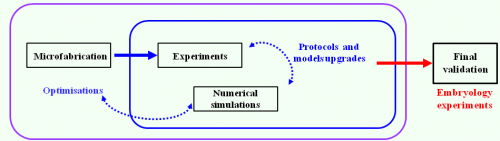
Figure 3. Interdisciplinary and auto-optimised strategy used to conceive a dielectric guide-based tool to electroporate locally, reproducibly, and safely small cells populations from external tissues of embryos or organ explants.
Bibliography
Calegari, F., Marzesco, A., Kittler, R., Buchholz, F., & Huttner, W. (2004). Tissue-specific RNA interference in post-implantation mouse embryos using directional electroporation and whole embryo culture Differentiation, 72 (2-3), 92-102 DOI: 10.1111/j.1432-0436.2004.07202002.x
Davidson, B., Tsang, T., Khoo, P., Gad, J., & Tam, P. (2003). Introduction of cell markers into germ layer tissues of the mouse gastrula by whole embryo electroporation genesis, 35 (1), 57-62 DOI: 10.1002/gene.10166
Escoffre, J., Portet, T., Wasungu, L., Teissié, J., Dean, D., & Rols, M. (2009). What is (Still not) Known of the Mechanism by Which Electroporation Mediates Gene Transfer and Expression in Cells and Tissues Molecular Biotechnology, 41 (3), 286-295 DOI: 10.1007/s12033-008-9121-0
Fox, M., Esveld, D., Valero, A., Luttge, R., Mastwijk, H., Bartels, P., Berg, A., & Boom, R. (2006). Electroporation of cells in microfluidic devices: a review Analytical and Bioanalytical Chemistry, 385 (3), 474-485 DOI: 10.1007/s00216-006-0327-3
Itasaki N, Bel-Vialar S, & Krumlauf R (1999). ‘Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nature cell biology, 1 (8) PMID: 10587659
Kim, J., Cho, K., Shin, M., Lee, W., Jung, N., Chung, C., & Chang, J. (2008). A novel electroporation method using a capillary and wire-type electrode Biosensors and Bioelectronics, 23 (9), 1353-1360 DOI: 10.1016/j.bios.2007.12.009
Mazari, E., Zhao, X., Migeotte, I., Collignon, J., Gosse, C., & Perea-Gomez, A. (2014). A microdevice to locally electroporate embryos with high efficiency and reduced cell damage Development, 141 (11), 2349-2359 DOI: 10.1242/dev.106633
Momose, T., Tonegawa, +., Takeuchi, J., Ogawa, H., Umesono, K., & Yasuda, K. (1999). Efficient targeting of gene expression in chick embryos by microelectroporation Development, Growth and Differentiation, 41 (3), 335-344 DOI: 10.1046/j.1440-169X.1999.413437.x
Nakamura, H., & Funahashi, J. (2013). Electroporation: Past, present and future Development, Growth & Differentiation, 55 (1), 15-19 DOI: 10.1111/dgd.12012
Soares, M., Torres-Padilla, M., & Zernicka-Goetz, M. (2008). Bone morphogenetic protein 4 signaling regulates development of the anterior visceral endoderm in the mouse embryo Development, Growth & Differentiation, 50 (7), 615-621 DOI: 10.1111/j.1440-169X.2008.01059.x
Swartz, M., Eberhart, J., Mastick, G., & Krull, C. (2001). Sparking New Frontiers: Using in Vivo Electroporation for Genetic Manipulations Developmental Biology, 233 (1), 13-21 DOI: 10.1006/dbio.2001.0181
Takaoka, K., & Hamada, H. (2012). Cell fate decisions and axis determination in the early mouse embryo Development, 139 (1), 3-14 DOI: 10.1242/dev.060095
Wang, M., Orwar, O., Olofsson, J., & Weber, S. (2010). Single-cell electroporation Analytical and Bioanalytical Chemistry, 397 (8), 3235-3248 DOI: 10.1007/s00216-010-3744-2
Wang, S., & Lee, L. (2013). Micro-/nanofluidics based cell electroporation Biomicrofluidics, 7 (1) DOI: 10.1063/1.4774071


 (No Ratings Yet)
(No Ratings Yet)