Exploring Mammary Gland Development and Evolution with Organoid Technology
Posted by Gat Rauner, on 6 February 2024
What is the set of instructions that directs cells as they form a tissue, and how did this set of instructions evolve throughout species evolution? In a new study, we generated organoids from a variety of species that can be utilized to answer these questions and more.

Organoids are miniature versions of an organ, grown in a 3D tissue culture. To understand the process of development and regeneration, our lab uses organoids. Our lab uses organoids that are generated from single cells and develop into architecturally and cellularly complex tissues. This model offers a valuable window into the developmental process.
Our model organ is the mammary gland, one of the most uniquely regenerative tissues and a hallmark of all mammals. This remarkable organ develops and regenerates mostly postnatally. During the embryonic period, a rudimentary network of epithelial ducts is established and remains dormant until puberty, when it expands and forms a ductal-lobular network. After the pubertal expansion, the mammary gland is capable of further maturation and expansion upon pregnancy and lactation, when it will mature to its full capacity as a milk-producing organ.
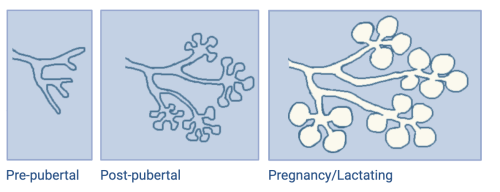
Unlike other organs that consistently regenerate, like the skin and intestinal epithelia, the mammary gland undergoes cycles of regeneration and degeneration but those are not pre-determined or necessarily continuous, they depend on specific hormonal cues and can be many years apart. As such, the mammary gland exists in a state of readiness to fulfill its full developmental capacity.
Remarkably, despite years of research, we are still unclear about the identity of the cells that regenerate the mammary gland, and how exactly they do it. Organoids offer the opportunity to observe mammary tissue formation from single cells in an experimental setting under controlled conditions.
Mammary organoid technology was originally developed for human breast tissue, where it aims to be an alternative to in-vivo experiments that we can’t do in humans. But if organoids can be an alternative to human tissue, perhaps they can also be an alternative to the many other mammals we cannot study.
Questions like: how did the mammary gland emerge during evolution? What regeneration mechanism did the mammary gland develop throughout? What is the precursor gland that the mammary gland evolved from? If the mammary gland evolved from a coiled apocrine gland, as is currently thought, when and how did the branching of the gland appear during evolution? How did gradual genetic changes lead to the wide variety of phenotypes manifested across mammals today? These questions and more can be studied by exploring the mammary glands of mammalian groups that diverged early in mammalian evolution, such as monotremes and marsupials.
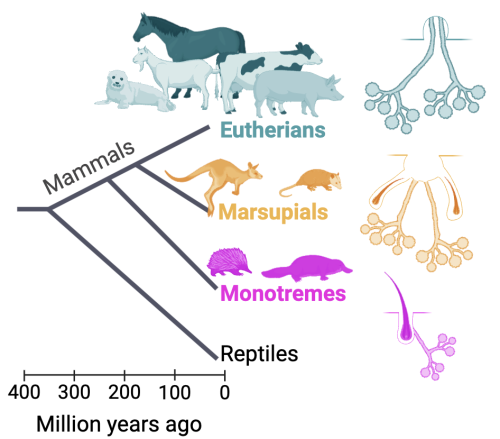
Monotremes, which diverged from eutherians about 190 million years ago, are mammals that lay eggs and then lactate the hatched offspring. Their mammary gland is primitive and is akin to a sweat gland associated with a hair follicle. Monotremes consist of only two species: the platypus and the echidna, both with restricted global spread and much fewer individuals compared to other mammalian groups. Marsupials, which diverged from eutherians about 166 million years ago, have more evolved mammary glands, but some still retain the hair association before pubertal development (it disappears in the adult gland).
The limited access to these species can be overcome by using organoid models. The ability to manipulate these organoids genetically also opens the potential to explore how genetic changes may affect the phenotype within and between species, effectively simulating mammalian evolution.
Other questions that can be answered by exploring mammary gland development, using organoids, relate to the regulation of milk production and milk composition. For example, the simultaneous secretion of different milk compositions in the Tammar wallaby indicates that milk composition can be regulated at the local tissue level, but it is unclear how [1-4]. Some species exhibit delayed involution, which is the degeneration of the gland when lactation stops, and the mechanisms behind this delay are not entirely understood [5-7]. It may assist in exploring questions related to breast cancer, as some mammals seem to be resistant to this type of cancer while others are highly susceptible [8].
To lay the foundations for exploring these questions, we sought to grow mammary gland organoids from single primary cells derived from 9 different species: 8 eutherian species (aka placental mammals), and one marsupial – the gray short-tailed opossum.
It took some trial and error (aka the “heuristic approach”) to reach culture conditions that support the growth of mammary organoids from all these species. In the process, we found that some but not all species organoids required inhibition of the ROCK protein to form branches in 3D culture. The mechanisms behind the involvement of ROCK proteins in branching are still unclear, but a model system of organoids from species that require the inhibitor and those that do not may help shed light on how ROCK proteins participate in branching morphogenesis.
In conclusion, our exploration of mammary gland development across various species using organoids offers a pioneering view into the evolution, development, and function of this unique organ. Mammary organoids stand as a versatile and powerful tool and will hopefully guide novel approaches to studying tissue regeneration, milk production regulation, and cancer resistance.
References
1. Green, B., K. Newgrain, and J. Merchant, Changes in milk composition during lactation in the tammar wallaby (Macropus eugenii). Australian Journal of Biological Sciences, 1980. 33(1): p. 35-42.
2. Nicholas, K.R., Asynchronous dual lactation in a marsupial, the tammar wallaby (Macropus eugenii). Biochemical and Biophysical Research Communications, 1988. 154(2): p. 529-536.
3. Sharp, J.A., et al., The tammar wallaby: A marsupial model to examine the timed delivery and role of bioactives in milk. Gen Comp Endocrinol, 2017. 244: p. 164-177.
4. Wanyonyi, S.S., et al., The extracellular matrix locally regulates asynchronous concurrent lactation in tammar wallaby (Macropus eugenii). Matrix Biology, 2013. 32(6): p. 342-351.
5. Sharp, J.A., et al., Fur seal adaptations to lactation: insights into mammary gland function. Current topics in developmental biology, 2006. 72: p. 275-308.
6. Sharp, J.A., et al., The fur seal-a model lactation phenotype to explore molecular factors involved in the initiation of apoptosis at involution. J Mammary Gland Biol Neoplasia, 2007. 12(1): p. 47-58.
7. Sharp, J.A., C. Lefevre, and K.R. Nicholas, Lack of functional alpha-lactalbumin prevents involution in Cape fur seals and identifies the protein as an apoptotic milk factor in mammary gland involution. BMC Biol, 2008. 6: p. 48.
8. Munson, L. and A. Moresco, Comparative pathology of mammary gland cancers in domestic and wild animals. Breast Dis, 2007. 28: p. 7-21.


 (No Ratings Yet)
(No Ratings Yet)