How fish grew limbs and moved onto land
Posted by WHB_Masselink, on 25 July 2016
Fin to limb transition was a crucial evolutionary adaptation necessary for the vertebrate land invasion in the late Devonian around 400 MY ago. Without this key event, I would not be writing this blog, we as a species would definitely not exist, and neither would any other tetrapod. While the gross morphological changes for this process are fairly well understood, the cellular and molecular mechanism controlling these changes remained a total mystery. In our recent publication (Masselink et al, 2016) we identified a unique and novel lineage of cells within the pectoral fins that have been gradually lost throughout tetrapod evolution and regulate the fin to limb transition by modulating FGF8 and FGF24 expression in the Apical Ectodermal Ridge (AER) and the underlying fin bud mesenchyme.
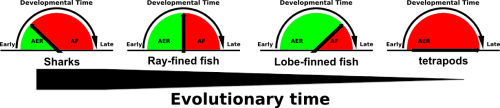
Comparative embryological studies by Thorogood and others suggested that a gradual delay and eventual complete disappearance of the AER to Apical Fold (AF) transition was a driving force of evolutionary fin to limb transition (figure 1, Thorogood, 1991). More recent work by Yano experimentally confirms Thorogood’s hypothesis (Yano, 2012). The Apical Ectodermal Ridge, an ectoderm derived structure that covers the underlying mesenchyme of both the fin and limb bud, secretes pro-proliferative factors such as FGF8 to provide cues for distal outgrowth of the underlying mesenchyme. While in tetrapods the AER perdures and eventually undergoes apoptosis, in fish the AER transitions into the AF, which eventually gives rise to the fin paddle and are populated by actinotrichia, non-ossifying collagen ridge fibrils which provide rigidity to the embryonic fin paddle. Thus a gradual delay and eventual complete disappearance of AER to AF transition would not only allow for the disappearance of the fin paddle but the prolonged presence of the AER would also allow for increased distal outgrowth of the underlying mesenchyme.
We identified a novel lineage of fish-specific AER infiltrating cells that control AER to AF transition that also secrete the collagens required for actinotrichia formation (figure 2). We have thus termed these cells Apical Fold Inducing Cells (AFICs). Using 3 different methods (confocal time lapse microscopy, photoconversions, and somite transplantation) we conclusively show these cells have a somitic origin, a lineage which despite decades of lineage tracing experiments was never identified in tetrapods, suggesting that this is indeed a process unique to fish. Metronidazole and laser mediated ablation were used to selectively ablate AFICs, resulting in fins to form with certain limb-like characteristics. Specifically we observed phenotypes consisting of an expanded mesenchyme, a disruption in AF formation, and a loss of actinotrichia, we also observed an increased expression of FGF8 in the AER and a delay of FGF24 translocation from the mesenchyme to the AER. Inhibition of FGF signalling by treatment with the FGF inhibitor SU5402 completely blocks the increased proliferative response in the mesenchyme, and thus provides a molecular basis for the fin to limb transition.
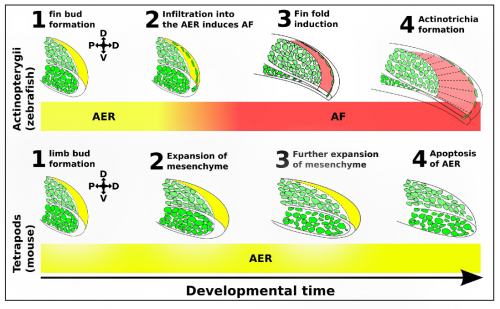
The induction of the fin fold and actinotrichia formation are two distinct processes, both controlled by AFICs. We have shown AFICs to consistently localize to the distal edge of the actinotrichia, and secrete actinotrichia-associated collagens. The loss of actinotrichia -by knockdown of Actinoidin 1 and 2- results in a fin fold defect but not an expansion of the underlying mesenchyme (Zhang, 2010). Thus while AFICs are essential for actinotrichia formation they play a second role in blocking the expansion of the fin bud mesenchyme, likely through fin fold induction and the subsequent modulation of FGF8 and FGF24.
To interrogate the function of AFICs in key phylogenetic representatives of the jawed vertebrate (gnathostome) lineage, we took advantage of the Collagen1a1a antibody that detects zebrafish AFICs. The extant gnathostome lineage is broadly made up of sharks, ray-finned fish and lobe-finned fish. Species within each group were selected based on availability. An active breeding facility of the small benthic Epaulette shark was established providing access to embryonic tissue, while frozen tissue sections of embryos from both the American paddlefish (basal ray-finned species) and the Australian lungfish (lobe-finned species) were fortunately available. We found that both the timing of residency and number of AFICs are gradually reduced throughout evolutionary representatives. While the epaulette shark contains a large number of AFICs in the AER at an early stage of embryonic development, this is already delayed in timing and reduced in number in the American paddlefish. Most strikingly the Australian lungfish, a species that abuts the tetrapod lineage contains very few AFICs, which infiltrate at a late stage, and are lost all together at later stages of development. The total number of AFICs and their timing of residence is in line with the phylogenetic sampling these species present and support Thorogood’s clock model.
While our results reinforce the validity of Thorogood’s clock model, and provide a cellular framework for the changes that had to occur during fin to limb transition, they open many new questions. What controls AFIC infiltration, and what controls its timing? At this point we can only speculate. AFICs migrate in a pool of muscle progenitors into the embryonic fin bud. Here they migrate through the basement membrane into the AER. While active migration through the basement membrane is very likely to be important, this does not in and of itself explain the temporal differences observed between species. Muscle progenitors are spread throughout the zebrafish fin bud mesenchyme, coming into direct contact with the basement membrane that separates the AER from the underlying mesenchyme. In contrast, in both chicken and mice the muscle progenitors in early limb buds are restricted to the proximal region of the mesenchyme and do not come into direct contact with the basement membrane. As such, it is possible to imagine that the time between fin/limb field establishment, and muscle delamination and subsequent migration might be essential.
References:
- Masselink, W. et al. A somitic contribution to the apical ectodermal ridge is essential for fin formation. Nature doi:10.1038/nature18953 (2016)
- Thorogood, P. in Developmental Patterning of the Vertebrate Limb (eds Hinchliffe, J. R. et al.) 347-354 (Springer US, 1991).
- Yano, T., Abe, G., Yokoyama, H., Kawakami, K. & Tamura, K. Mechanism of pectoral fin outgrowth in zebrafish development. Development 139, 2916-2925 (2012).
- Zhang, J. et al. Loss of fish actinotrichia proteins and the fin-to-limb transition. Nature 466, 234-237 (2010).


 (8 votes)
(8 votes)