Gastrulation in a Dish
Posted by Hailey Chitrin, on 12 December 2022
This summer, I was under the supervision of Ashley Libby in James Briscoe’s Lab at the Crick, where they are interested in studying how the spinal cord forms before birth. The lab uses the spinal cord as a model to understand general concepts in embryonic development; research that when better understood can be invaluable in areas such as regenerative medicine. The main aim of my project was to design an in vitro system to study chicken gastrulation.
Embryonic stem cells(ESCs) derived from the blastula of early-stage embryos have the ability to differentiate and give rise to all cell types. Gastruloids are three-dimensional aggregates of ESCs that recapitulate aspects of the organisation of a gastrulating embryo. Gastrulation is one of the most important organising events in the embryo and is responsible for germ layer specification and axis organisation. For this reason, gastruloids are a useful tool for studying cell type emergence in vitro and dissecting unknown aspects of the processes that direct development. However, there is still a lot of research to be carried out to better understand aspects of cell organisation, communication, and signalling for gastruloids to better resemble their target form.
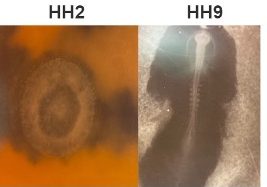
My project began with the exploration of other gastruloid studies in mice, zebrafish, and humans, to develop a method that could be used to replicate this in other animal models. We are able to use such a wide range of models to study gastrulation in humans because of the specific time point in development where embryos of many species share a biological resemblance to each other. The model I tested was the chicken embryo (Figure 1). The first few weeks of my time spent at the Crick involved learning how to dissect the chick embryo and learning how to identify the different ages of each embryo. This process involved practising how to use microscope techniques to harvest embryos.
To achieve my end goal of generating a gastruloid, we decided to test Hamburger Hamilton stage 2 (HH2) and Hamburger Hamilton stage 9 (HH9) to provide us with two different stem cell pools. Cells from HH2 embryos have the capacity to differentiate into all cell types. However, the slightly older HH9 embryo has started to differentiate some of its cells but still provides us with the caudal lateral epiblast which we know to be responsible for spinal cord and somite formation driving body axis elongation. My experiments over the summer focused on comparing these two stem cell pools to determine which one is more effective for cell re-organisation following dissociation.
We designed a protocol to first dissect HH2 embryos or the caudal epiblast from HH9 embryos. Then dissociate the two dissected tissues into a single cell suspension before adding them to a non-adherent 96-well plate.
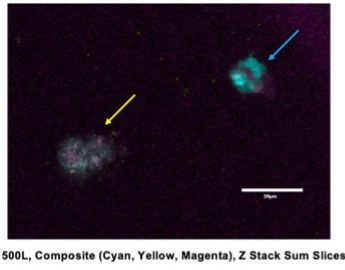
To determine both cell viability and whether organisation occurs within our aggregates, we stained the cells with primary and secondary antibodies for the presence of Brachyury and Sox2. Brachyury is a transcription factor expressed in the developing notochord and primitive streak, whereas Sox2 is a transcription factor required for neural development. We expected that if organization and elongation had occurred, Brachyury and Sox2 would be present in separate domains of the cell aggregate. However, of the various conditions I tested, none were effective in allowing for cell viability for 5 days post-embryo dissociation. This was evident by a lack of fluorescent staining with DAPI that would indicate the presence of DNA, overlapping Sox2 and Brachyury, and difficulty finding real cells that had aggregated and grown after fixing and staining (Figure 2). This raised a lot of questions about what changes needed to be made to make a chicken gastruloid.
Overall, this summer has been a fantastic learning experience for myself. Being able to work with my supervisor Ashley Libby and learning from her has helped my confidence in the lab and my ability to understand scientific content. Working in the Briscoe Lab has been very enjoyable and has encouraged me to consider a career in research- something I wasn’t as interested in before this placement. For this reason, I would also like to thank the Francis Crick Institute for giving me the chance to carry out this project and also the MRF Rosa Beddington Fund for supporting my project.

If interested, here is a link to James Briscoes Lab.
You can contact me through LinkedIn: https://www.linkedin.com/in/hailey-chitrin


 (5 votes)
(5 votes)