Geometry first: how positional cues dictate fate in bilayered epithelia
Posted by Robin Journot, on 4 September 2025
Overview
In four murine bilayered epithelia, the first 3D architectural transition—cell internalization during placode formation—triggers symmetry breaking. YAP reads the cell position; Notch commits neighbors to basal vs luminal fates1.
How the project started
This project began with a simple observation inspired by our lab’s work on early-stage mammary gland development2: even before branching morphogenesis, cell fate is already spatially organized in the embryonic mammary gland. This robust patterning, with distinct cell lineages emerging so early, prompted us to ask which pathways underlie such fate decisions. The idea to broaden our scope beyond the mammary gland arose unexpectedly, when scRNA-seq of an embryonic mammary sample revealed an additional “contaminating” cell population. Analyzing this population uncovered striking similarities with other tissues, sparking the multi-organ perspective that eventually shaped the project.
Why these tissues?
We chose to study the mammary, lacrimal, salivary glands, and prostate for three reasons:
- they share a bilayered architecture and the same cellular hierarchy with stem cells giving rise to basal and luminal cells,
- during fate specification they show similar transcriptional signatures and dynamic of fate potency restriction, and
- they’re all branched epithelia.
In other words, different organs with distinct embryonic origins, but a common structural logic, thus the perfect experimental paradigm for testing whether a conserved mechanism underlies early tissue compartmentalization and fate segregation.
What we learned
In both organoids and embryonic tissue explants, symmetry breaking coincided with cell internalization: internal cells acquire high Notch activity (HES1) while external cells retain p63 expression and nuclear YAP (Figure 1). YAP acts as the position interpreter—it is uniform in all cells before internalization/tissue compartmentalization, while it becomes spatially restricted afterward. On the other hand, Notch acts as the commitment machinery, necessary and sufficient to drive luminal cell identity. Perturbations that enforce uniform YAP activity hold cells in a hybrid p63⁺/HES1⁺ state and delay tissue compartmentalization, while activating Notch overrides that block in differentiation and imposes luminal fate acquisition.
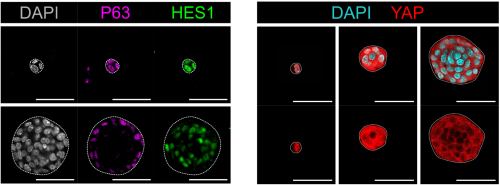
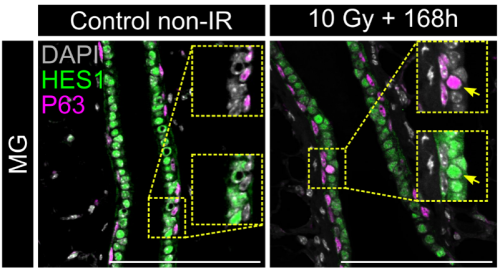
In adult tissue regeneration (induced by luminal-cell ablation or irradiation), we observed the same hybrid p63⁺/HES1⁺ cells and an increase of cells harboring nuclear YAP, rekindling the pre-committed state in early development. The critical tissue size at which symmetry breaking occurs is bigger in vivo than in organoids, likely because niche inputs modulate YAP signaling, delaying cell commitment despite similar geometry.
A conserved “hourglass” logic
Despite distinct origins (ectoderm-derived exocrine glands vs. endoderm-derived prostate), these organs appear to reuse a common core toolkit at the point of symmetry breaking and stem cell commitment. Diverse upstream inputs including geometry, niche and tissue mechanics, converge on YAP at the bottleneck of an hourglass, which then gates Notch–p63 interactions to resolve fate. Later in development, tissues diverge again by following organ-specific programs tailored to the different functions of each tissue. The high conservation of this middle bottleneck, YAP → Notch/p63, is what gives the mechanism both robustness and portability across different contexts, including regeneration.
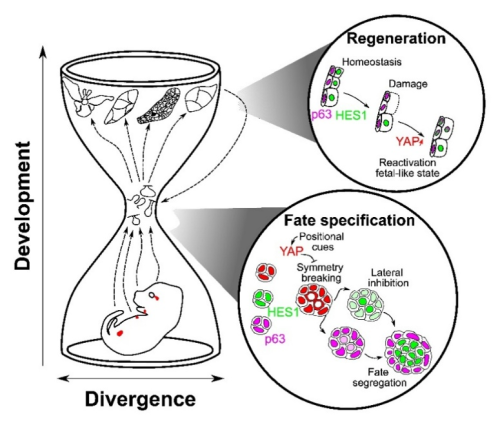
Take-home
Tissue architecture initiates, YAP interprets, Notch resolves. Cell internalization acts as the deterministic cue that converts tissue shape into cell fate across bilayered epithelia—and the same logic is redeployed during repair.
1. Journot, R.P., Huyghe, M., Barthelemy, A., Couto-Moreira, H., Deshayes, T., Harari, L., Sumbal, J., Faraldo, M.M., Dubail, M., Fouillade, C., et al. (2025). Conserved signals control self-organization and symmetry breaking of murine bilayered epithelia during development and regeneration. Dev. Cell. https://doi.org/10.1016/j.devcel.2025.06.007.
2. Carabaña, C., Sun, W., Veludo Ramos, C., Huyghe, M., Perkins, M., Maillot, A., Journot, R., Hartani, F., Faraldo, M.M., Lloyd-Lewis, B., et al. (2024). Spatially distinct epithelial and mesenchymal cell subsets along progressive lineage restriction in the branching embryonic mammary gland. EMBO J., 1–29. https://doi.org/10.1038/s44318-024-00115-3.


 (3 votes)
(3 votes)