How do jellyfish regenerate their tentacles?
Posted by Yuichiro Nakajima, on 27 May 2024
In the paper “Distinct stem-like cell populations facilitate functional regeneration of the Cladonema medusa tentacle”, Fujita and colleagues found that the mechanism of tentacle regeneration in jellyfish is more similar to how salamanders regenerate their limbs than how other cnidarians regenerate. First author Sosuke Fujita and corresponding author Yuichiro Nakajima tell us more about the story behind the paper.
How did the project get started?
It has long been known that jellyfish organs like the manubrium and the tentacle can regenerate, but the detailed mechanisms at the cellular and molecular levels remain unclear. Using the hydrozoan jellyfish Cladonema pacificum (Figure 1), we began this study by examining the regenerative potential of each organ and found that tentacles have a high regenerative capacity, functionally regenerating within 2-3 days, which exceeded our expectations. We anticipated that the Cladonema tentacle would be a useful model for studying appendage regeneration and aimed to comprehensively understand its mechanism. To this end, we decided to monitor regeneration processes including wound healing, cell proliferation, stem cell distribution, and the timing of cell differentiation over time. In particular, we found that cell proliferation occurs intensively at the site of injury in the early phase of regeneration after wound healing completes. Based on these observations, we asked the simple question, “How do proliferating cells appear at the site of injury?”

What is known about blastema formation during regeneration before your work?
Blastema is an aggregate of cells that includes undifferentiated cells like stem cells, serving as a cellular source for regeneration. Blastema formation is a critical step for regeneration, occurring in regenerative animals like planarians, salamanders, and cnidarians, which are among the early-branching animals. However, methods of blastema formation, particularly the way of supplying undifferentiated cells, vary among regenerative animals, and the evolutionary aspects of the process remain unknown. While it is ideal to understand the mechanism of blastema formation across diverse animals, our knowledge about it in basal metazoans is mainly limited to a few polyp-type models, such as Hydra and Hydractinia (Bradshaw et al., 2015; Chera et al., 2009). We thus focused our attention on blastema formation during organ/appendage regeneration in jellyfish. Understanding this mechanism in jellyfish allows us to compare it with cnidarian polyps as well as some regenerative bilaterians.
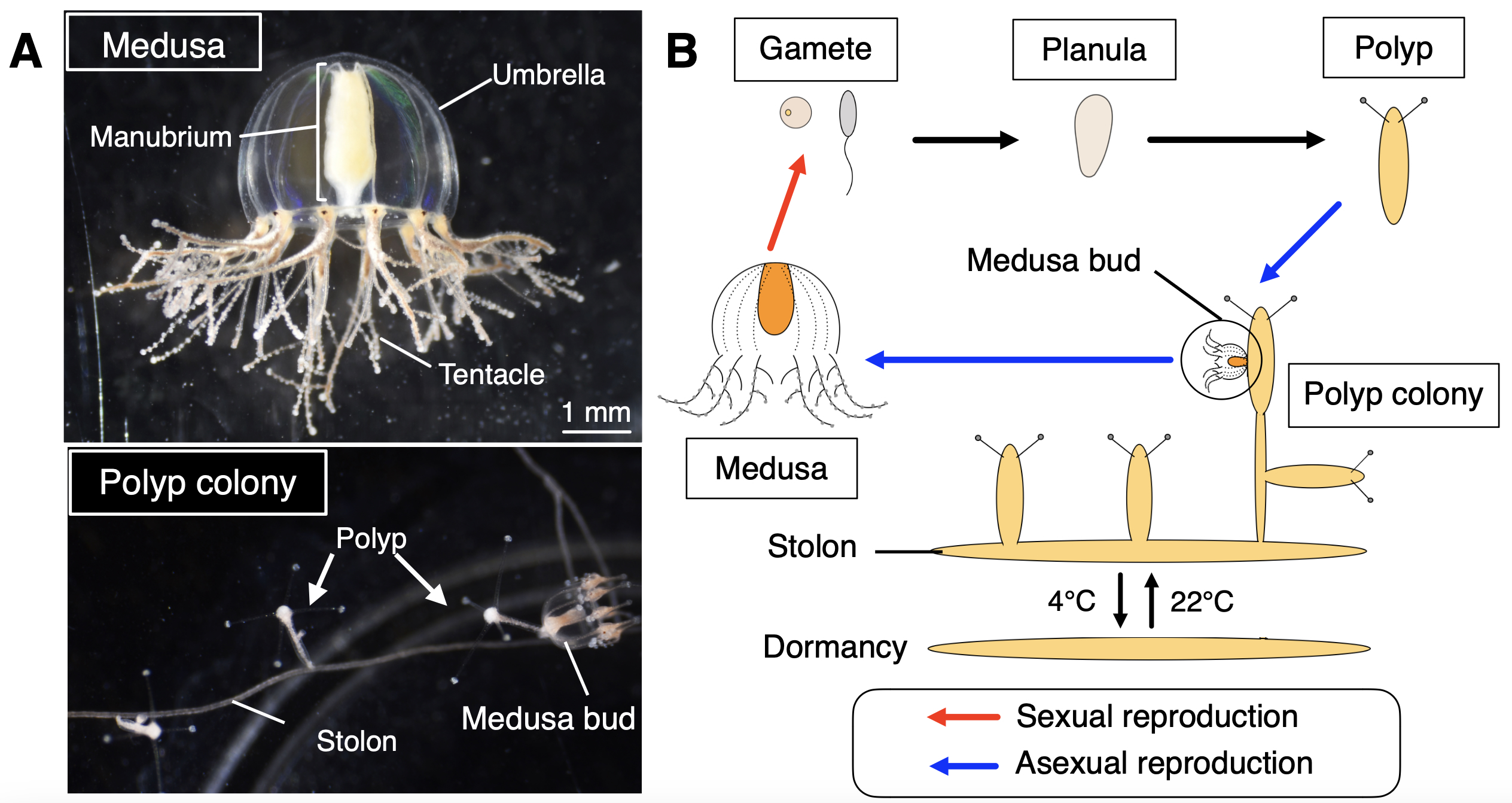
Why did you choose the jellyfish Cladonema pacificum as your research organism?
Cladonema pacificum is a small jellyfish (less than 1 cm in height) widely found along the coast of Japan with a typical hydrozoan life cycle including the polyp and medusa stages (Figure 2). The Cladonema medusa is basically benthic, and all the stages are easily kept in small containers without any special equipment. This organism can be stored at 4°C for years as stolons (structures connecting polyps) as a dormant stage, which can reproduce polyps and medusae once returned to a warm temperature (e.g., 22°C), making maintenance easier. We are able to keep this jellyfish in our laboratory, which is located far from the sea. Importantly, Cladonema has the ability to regenerate organs, especially lost tentacles, in just a few days (Fujita et al., 2019). These factors made Cladonema an ideal model for studying organ regeneration in jellyfish.
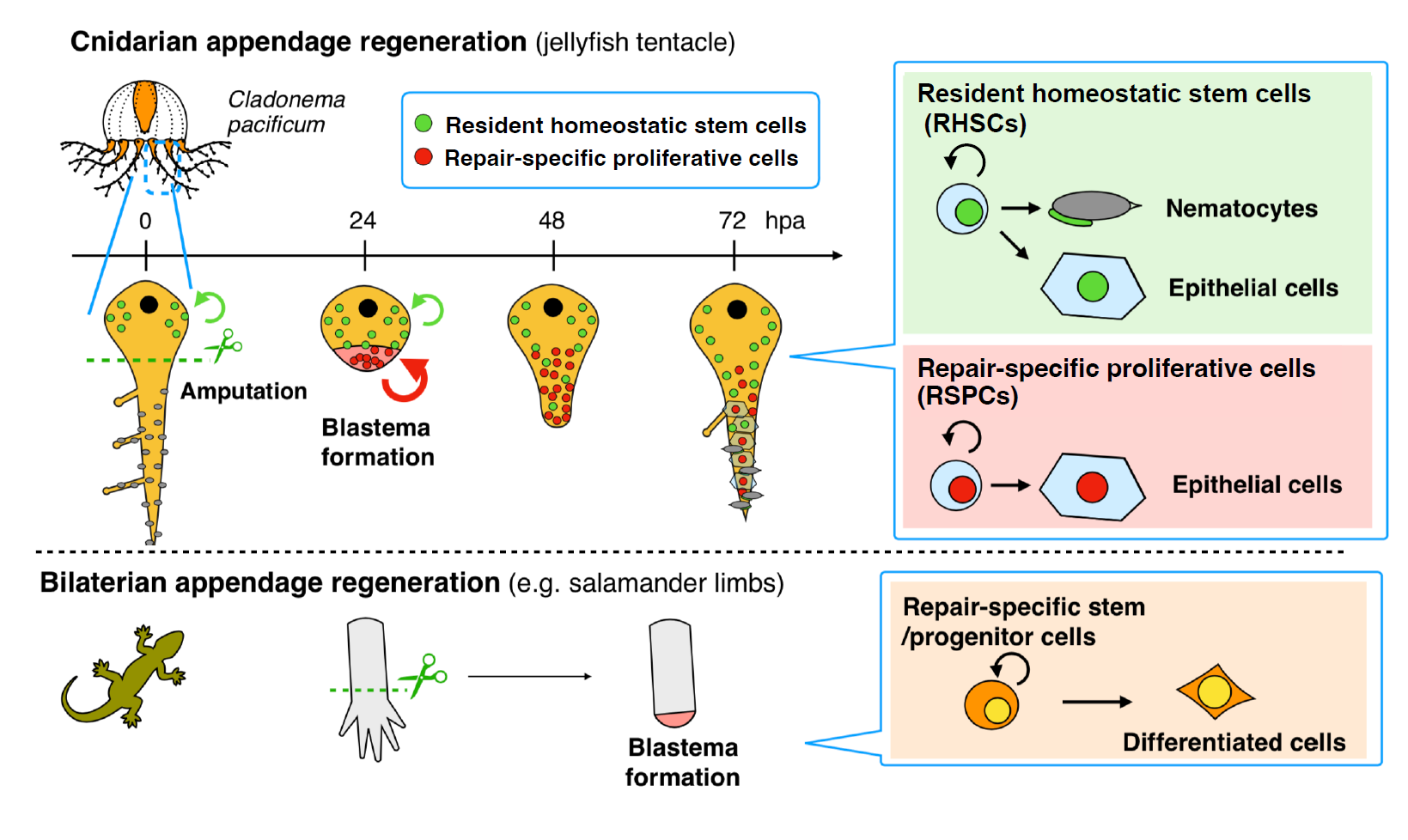
Can you summarize the key findings of the paper in one paragraph?
Based on previous studies using polyp models, blastema formation in cnidarians was thought to utilize the recruitment of resident pluripotent/multipotent stem cells. In contrast, we found that repair-specific proliferative cells (RSPCs) with stem cell characteristics appear upon amputation and are mainly involved in blastema formation during tentacle regeneration. Additionally, RSPCs preferentially differentiate into epithelial cells in the newly regenerating tentacle, while resident stem cells supply all the cell types including cnidocytes, promoting functional tentacle regeneration. These results suggest that blastema formation during Cladonema tentacle regeneration is analogous to limb regeneration in newts, rather than cnidarian polyps (Figure 3; Fujita et al., 2023). Our findings imply a common mechanism for supplying repair-specific blastema cells for complex organ regeneration that has independently evolved in each animal phylum.
Were you surprised to find that most blastema cells do not come from resident stem cells but instead from repair-specific proliferative cells (RSPCs)?
Yes, we were surprised. We had initially expected resident stem cells to migrate and form blastema as seen in other cnidarian polyp systems during whole-body regeneration. We were puzzled when the nucleoside chase experiments showed unexpected results. We thus needed additional evidence to clarify this: for example, we found that RSPCs can still appear even after eliminating resident stem cells with X-ray irradiation or drugs. During the revision process of the paper (Fujita et al., 2023), we addressed numerous concerns related to this issue with further experiments, which strengthened our observations.
Can you postulate the cellular origins of the RSPCs?
Yes, we believe there are at least two possibilities: one is the reactivation of quiescent stem cells and the other is dedifferentiation. Similar to satellite cells in vertebrate muscle, damage can reactivate their cell cycle to become RSPCs, giving rise to differentiated cells. Alternatively, upon injury stimulus, differentiated cells can revert to RSPCs with an undifferentiated state via dedifferentiation, becoming able to proliferate. We are trying to figure out which is the case for tentacle regeneration.
Were there any other unexpected results and challenges, especially associated with working with a non-model research organism?
Working with non-model animals presented various challenges. Existing protocols for other models, even cnidarians, often require modification, and establishing experimental methods can be time-consuming (Fujita et al., 2022; Masuda-Ozawa et al., 2022). Additionally, some common knowledge does not apply to specific animals and contexts: for instance, some marker genes are not expressed in expected cell types, and their expression can be heterogeneous among tissues and locations, which initially became an issue. After rounds of investigation and consideration, these unexpected results eventually provide insights into the differences between what we observe and what is already known. Overall, the experience of working with a non-model research organism has been more enjoyable than challenging.
What are the implications of your findings in understanding regeneration in other organisms?
Our findings (Figure 3) imply that blastema can be supplied by repair-specific stem cell populations, similar to those observed in salamander limb regeneration (Sandoval-Guzman et al., 2014), rather than by resident stem cells, which previous cnidarian studies had focused on (Amiel et al., 2019; Bradshaw et al., 2015; Chera et al., 2009). Furthermore, the heterogeneity of stem cells discovered in the context of regeneration might also be identified during development and homeostasis in cnidarians or basal metazoans. Investigating how stem cells contribute to regeneration and how blastema forms during regeneration across different cnidarians will enhance our understanding of the evolutionary trajectory of the blastema formation program.
What’s next for this story and your lab?
We want to understand how the two stem cell populations we identified are regulated differently during regeneration. Our lab is currently identifying the signaling pathways that control cell proliferation and differentiation in each cell population. We also aim to determine how RSPCs emerge, especially their cellular origin. To distinguish different possibilities, we are designing new experiments and developing genetic tools in Cladonema. These approaches will provide a better understanding of regenerative processes at the molecular level and enhance our ability to explore diverse phenomena in jellyfish.
References:
Amiel, A. R., Foucher, K., Ferreira, S. and Röttinger, E. (2019). Synergic coordination of stem cells is required to induce a regenerative response in anthozoan cnidarians. bioRxiv, 2019.2012.2031.891804. 10.1101/2019.12.31.891804
Bradshaw, B., Thompson, K. and Frank, U. (2015). Distinct mechanisms underlie oral vs aboral regeneration in the cnidarian Hydractinia echinata. Elife 4, e05506. 10.7554/eLife.05506
Chera, S., Ghila, L., Dobretz, K., Wenger, Y., Bauer, C., Buzgariu, W., Martinou, J. C. and Galliot, B. (2009). Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell 17, 279-289. 10.1016/j.devcel.2009.07.014
Fujita, S., Kuranaga, E., Miura, M. and Nakajima, Y. I. (2022). Fluorescent In Situ Hybridization and 5-Ethynyl-2′-Deoxyuridine Labeling for Stem-like Cells in the Hydrozoan Jellyfish Cladonema pacificum. J Vis Exp. 10.3791/64285
Fujita, S., Kuranaga, E. and Nakajima, Y. I. (2019). Cell proliferation controls body size growth, tentacle morphogenesis, and regeneration in hydrozoan jellyfish Cladonema pacificum. PeerJ 7, e7579. 10.7717/peerj.7579
Fujita, S., Takahashi, M., Kumano, G., Kuranaga, E., Miura, M. and Nakajima, Y. I. (2023). Distinct stem-like cell populations facilitate functional regeneration of the Cladonema medusa tentacle. PLoS Biol 21, e3002435. 10.1371/journal.pbio.3002435
Masuda-Ozawa, T., Fujita, S., Nakamura, R., Watanabe, H., Kuranaga, E. and Nakajima, Y. I. (2022). siRNA-mediated gene knockdown via electroporation in hydrozoan jellyfish embryos. Sci Rep 12, 16049. 10.1038/s41598-022-20476-1
Sandoval-Guzman, T., Wang, H., Khattak, S., Schuez, M., Roensch, K., Nacu, E., Tazaki, A., Joven, A., Tanaka, E. M. and Simon, A. (2014). Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 14, 174-187. 10.1016/j.stem.2013.11.007


 (2 votes)
(2 votes)