In Development this week (Vol. 140, Issue 4)
Posted by Seema Grewal, on 29 January 2013
Here are the highlights from the current issue of Development:
Pancreatic injury unlocks cell potential
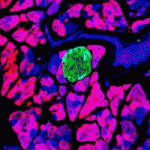 Identifying methods by which pancreatic β-cells can be produced is of major therapeutic importance. Whether there are adult pancreatic cells with the potential to make new β-cells is a matter of much debate. During embryonic development, the transcription factor Ptf1a initially marks multipotent progenitors, before becoming restricted to acinar cells. Here (p. 751), Christopher Wright and colleagues test whether mature Ptf1a-expressing cells can regain multipotentiality upon injury by labelling Ptf1a-positive acinar cells in mice and following their fate after pancreatic duct ligation. Remarkably, not only do new duct cells arise from the labelled cells, but some labelled cells start to express endocrine markers and display the hallmarks of mature β-cells, suggesting transdifferentiation of acinar cells into β-cells. This process is inefficient and slow, but can be enhanced by prior ablation of endogenous β-cells. Thus, pancreatic injury appears to induce reactivation of a more embryonic-like multipotent state in Ptf1a-expressing cells, from which endocrine cells can differentiate, possibly opening up new avenues for generating β-cells.
Identifying methods by which pancreatic β-cells can be produced is of major therapeutic importance. Whether there are adult pancreatic cells with the potential to make new β-cells is a matter of much debate. During embryonic development, the transcription factor Ptf1a initially marks multipotent progenitors, before becoming restricted to acinar cells. Here (p. 751), Christopher Wright and colleagues test whether mature Ptf1a-expressing cells can regain multipotentiality upon injury by labelling Ptf1a-positive acinar cells in mice and following their fate after pancreatic duct ligation. Remarkably, not only do new duct cells arise from the labelled cells, but some labelled cells start to express endocrine markers and display the hallmarks of mature β-cells, suggesting transdifferentiation of acinar cells into β-cells. This process is inefficient and slow, but can be enhanced by prior ablation of endogenous β-cells. Thus, pancreatic injury appears to induce reactivation of a more embryonic-like multipotent state in Ptf1a-expressing cells, from which endocrine cells can differentiate, possibly opening up new avenues for generating β-cells.
Lipid leads the way in wound healing
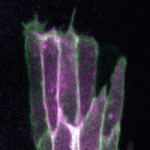 During epithelial wound healing, actin assembles at the leading edge of cells that border the wound, forming dynamic protrusions and, in some cases, an actomyosin cable. Together, these actin-rich structures are essential for wound closure. The process of dorsal closure in Drosophila shares many characteristics with wound healing and is a convenient system for cell biological analysis. Building on earlier results showing that the apical polarity determinant Par3/Bazooka (Baz) is lost from the leading edge of cells during dorsal closure, Tom Millard and colleagues (p. 800) now uncover a molecular mechanism by which Baz localisation regulates actin dynamics. Baz is known to bind the lipid phosphatase Pten, and the authors find that loss of Baz from the leading edge causes Pten redistribution. This, in turn, leads to an accumulation of the lipid PIP3 at the leading edge, which promotes formation of actin protrusions that are required for closure. This pathway is conserved during both dorsal closure and wound healing, offering a mechanistic basis for actin assembly during epithelial closure.
During epithelial wound healing, actin assembles at the leading edge of cells that border the wound, forming dynamic protrusions and, in some cases, an actomyosin cable. Together, these actin-rich structures are essential for wound closure. The process of dorsal closure in Drosophila shares many characteristics with wound healing and is a convenient system for cell biological analysis. Building on earlier results showing that the apical polarity determinant Par3/Bazooka (Baz) is lost from the leading edge of cells during dorsal closure, Tom Millard and colleagues (p. 800) now uncover a molecular mechanism by which Baz localisation regulates actin dynamics. Baz is known to bind the lipid phosphatase Pten, and the authors find that loss of Baz from the leading edge causes Pten redistribution. This, in turn, leads to an accumulation of the lipid PIP3 at the leading edge, which promotes formation of actin protrusions that are required for closure. This pathway is conserved during both dorsal closure and wound healing, offering a mechanistic basis for actin assembly during epithelial closure.
Mapping the neural crest
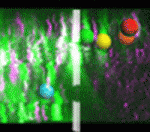 Neural crest (NC) cells arise in the neural tube (NT), undergo an epithelial-mesenchymal transition, and migrate away along defined routes, differentiating into multiple lineages. Precisely how NC cells exit the NT, and whether their fate is predetermined by their initial position within the NT, has been controversial. To address these issues, the Kulesa and Bronner laboratories performed a collaborative study (p. 820). Using a combination of photoactivation and two-photon time-lapse microscopy, they precisely marked individual or small groups of NC precursors in vivo in the chick embryonic NT and followed their fate. They found that most NC cells exit the NT at the dorsal midline, and that some precursors remain resident in the dorsal midline, producing an unordered emigration of cells. Moreover, they showed that differentiation potential is not defined by initial position within the NT, as has previously been suggested, although time of NT exit did influence fate. Together, these results suggest a more plastic and dynamic behaviour for NC cell emigration than previously appreciated.
Neural crest (NC) cells arise in the neural tube (NT), undergo an epithelial-mesenchymal transition, and migrate away along defined routes, differentiating into multiple lineages. Precisely how NC cells exit the NT, and whether their fate is predetermined by their initial position within the NT, has been controversial. To address these issues, the Kulesa and Bronner laboratories performed a collaborative study (p. 820). Using a combination of photoactivation and two-photon time-lapse microscopy, they precisely marked individual or small groups of NC precursors in vivo in the chick embryonic NT and followed their fate. They found that most NC cells exit the NT at the dorsal midline, and that some precursors remain resident in the dorsal midline, producing an unordered emigration of cells. Moreover, they showed that differentiation potential is not defined by initial position within the NT, as has previously been suggested, although time of NT exit did influence fate. Together, these results suggest a more plastic and dynamic behaviour for NC cell emigration than previously appreciated.
X inactivation: the great escape
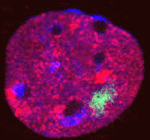 X-chromosome inactivation (XCI) enables dosage compensation between XX females and XY males, and its absence causes lethality, owing to defects in extra-embryonic tissues. However, it has also been shown that some genes are able to escape XCI in these tissues. Here, Catherine Corbel, Edith Heard and colleagues reconcile these findings and show that the inactive X (Xi) in one particular extra-embryonic cell type – trophoblast giant cells (TGCs) – has an unusual chromatin status (p. 861). Using RNA FISH on sections of postimplantation mouse embryos, they show that XCI is maintained in embryonic lineages, whereas TGCs show a high level of escape from XCI. Partial re-expression of most X-linked genes analysed, with the exception of the G6pd housekeeping gene, was observed in TGCs. In addition, the Xi in TGCs possesses an unusual organization and chromatin status, exhibiting both active and inactive chromatin marks. The authors propose that this apparent ‘bivalence’ of the Xi might account for its instability in TGCs and suggest that additional mechanisms maintain silencing at key loci.
X-chromosome inactivation (XCI) enables dosage compensation between XX females and XY males, and its absence causes lethality, owing to defects in extra-embryonic tissues. However, it has also been shown that some genes are able to escape XCI in these tissues. Here, Catherine Corbel, Edith Heard and colleagues reconcile these findings and show that the inactive X (Xi) in one particular extra-embryonic cell type – trophoblast giant cells (TGCs) – has an unusual chromatin status (p. 861). Using RNA FISH on sections of postimplantation mouse embryos, they show that XCI is maintained in embryonic lineages, whereas TGCs show a high level of escape from XCI. Partial re-expression of most X-linked genes analysed, with the exception of the G6pd housekeeping gene, was observed in TGCs. In addition, the Xi in TGCs possesses an unusual organization and chromatin status, exhibiting both active and inactive chromatin marks. The authors propose that this apparent ‘bivalence’ of the Xi might account for its instability in TGCs and suggest that additional mechanisms maintain silencing at key loci.
HNF1β controls nephron development
The nephron is a highly specialised unit of the kidney. It arises by mesenchymal-to-epithelium transitions. After epithelialization, a polarized renal vesicle forms, and this further differentiates into a comma-shaped body and a S-shaped body (SSB), in which the future nephron segments are mapped into proximal, intermediate and distal domains. How SSBs are patterned and subsequently differentiate during kidney morphogenesis is poorly defined. Here, two papers use complementary approaches to show that hepatocyte nuclear factor 1β (HNF1β), which is known to be required for the earliest steps of metanephric kidney development and is implicated in developmental renal pathologies, controls this early patterning.
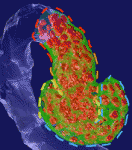 On p. 873, Silvia Cereghini and co-workers show that conditional inactivation of Hnf1b in murine nephron progenitors causes abnormal SSB regionalisation and morphology. In particular, Hnf1b deficiency leads to the absence of a proximal-medial SSB subdomain. This defect correlates with a downregulation of Notch pathway components and of Iroquois transcription factors, and perturbs the subsequent differentiation and morphogenesis of SSBs. Using parallel studies in Xenopus embryos, the researchers show that Hnf1b is required for the acquisition of proximal and intermediate tubule fate, acting again through the Notch pathway and Iroqouis genes. Together, these results show that HNF1B is required for the acquisition of a proximal-medial segment fate in vertebrates and uncover a previously unappreciated function of a novel SSB subdomain.
On p. 873, Silvia Cereghini and co-workers show that conditional inactivation of Hnf1b in murine nephron progenitors causes abnormal SSB regionalisation and morphology. In particular, Hnf1b deficiency leads to the absence of a proximal-medial SSB subdomain. This defect correlates with a downregulation of Notch pathway components and of Iroquois transcription factors, and perturbs the subsequent differentiation and morphogenesis of SSBs. Using parallel studies in Xenopus embryos, the researchers show that Hnf1b is required for the acquisition of proximal and intermediate tubule fate, acting again through the Notch pathway and Iroqouis genes. Together, these results show that HNF1B is required for the acquisition of a proximal-medial segment fate in vertebrates and uncover a previously unappreciated function of a novel SSB subdomain.
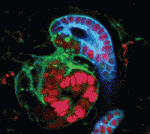 Using a similar gene targeting approach, Evelyne Fischer and colleagues (p. 886) demonstrate that Hnf1b inactivation in the murine metanephric mesenchyme (MM), which gives rise to nephron progenitors, leads to drastic tubular defects. The researchers report that mutant embryos show significant alterations to SSB structure: the typical bulge of epithelial cells between the intermediate and distal SSB segments is absent in mutant embryos. The lack of Hnf1b correlates with decreased expression of several genes, including the Notch ligand Delta-like 1, and results in impaired tubular expansion and differentiation. Finally, the researchers show that the nephron defects observed in Hnf1b-deficient mice resemble those observed in human foetuses carrying HNF1B mutations. The authors conclude that HNF1β plays an essential role in controlling the formation of a specific SSB sub-compartment by activating a set of crucial kidney development genes.
Using a similar gene targeting approach, Evelyne Fischer and colleagues (p. 886) demonstrate that Hnf1b inactivation in the murine metanephric mesenchyme (MM), which gives rise to nephron progenitors, leads to drastic tubular defects. The researchers report that mutant embryos show significant alterations to SSB structure: the typical bulge of epithelial cells between the intermediate and distal SSB segments is absent in mutant embryos. The lack of Hnf1b correlates with decreased expression of several genes, including the Notch ligand Delta-like 1, and results in impaired tubular expansion and differentiation. Finally, the researchers show that the nephron defects observed in Hnf1b-deficient mice resemble those observed in human foetuses carrying HNF1B mutations. The authors conclude that HNF1β plays an essential role in controlling the formation of a specific SSB sub-compartment by activating a set of crucial kidney development genes.
PLUS…
Stem cells living with a Notch
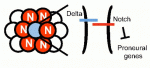 Freddy Radtke and colleagues review the role of Notch signaling in stem cells, comparing insights from flies, fish and mice to highlight similarities, as well as differences, between species, tissues and stem cell compartments. See the Review article on p. 689
Freddy Radtke and colleagues review the role of Notch signaling in stem cells, comparing insights from flies, fish and mice to highlight similarities, as well as differences, between species, tissues and stem cell compartments. See the Review article on p. 689
Human pluripotent stem cells: an emerging model in developmental biology
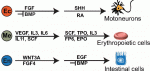 Zhu and Huangfu discuss how studies of human pluripotent stem cells (hPSCs) can complement classic approaches using model organisms, and how hPSCs can be used to recapitulate aspects of human embryonic development ‘in a dish’. See the Review on p. 705
Zhu and Huangfu discuss how studies of human pluripotent stem cells (hPSCs) can complement classic approaches using model organisms, and how hPSCs can be used to recapitulate aspects of human embryonic development ‘in a dish’. See the Review on p. 705


 (No Ratings Yet)
(No Ratings Yet)