In Development this week (Vol. 137, Issue 24)
Posted by Seema Grewal, on 23 November 2010
Pak1-ing a punch in lumen formation
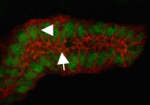
The generation and maintenance of correct lumen size and shape is essential for the function of tubular organs. Now, Monn Monn Myat and co-workers report that p21-activated kinase (Pak1) plays a novel role during lumen formation in Drosophila embryonic salivary glands (see p. 4177). The researchers show that Pak1 regulates the size and elongation of the apical domain of individual epithelial cells in the developing gland by decreasing and increasing E-cadherin levels at adherens junctions and basolateral membranes, respectively. Pak1 mediates these effects, they report, through Rab5- and Dynamin-dependent endocytosis of E-cadherin. Moreover, constitutively active Pak1 induces the formation of multiple intercellular lumens in the gland, an effect that is dependent on Rab5 and Dynamin, and on the Pak1 substrate Merlin. Together, these results identify a crucial role for Pak1 and E-cadherin endocytosis in lumen size and shape determination in fly salivary glands, and highlight a mechanism for multiple lumen formation, a process that occurs in pathological conditions such as breast ductal carcinoma in situ.
Shh signalling out-Foxed by cilia
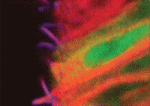
Sonic hedgehog (Shh) signalling controls cellular differentiation in the neural tube by regulating a poorly defined gene regulatory network. To better understand this network, James Briscoe and colleagues have undertaken a genome-wide expression screen in chick neural tube and, on p. 4271, they identify the forkhead transcription factor Foxj1 as an Shh target gene in this tissue. Foxj1, they report, is expressed in the chick and mouse neural tube in cells that constitute the floor plate (FP), a neural tube organising centre. Foxj1 expression is associated with the formation of long motile cilia in several cell types and, consistent with this, the authors show that chick and mouse FP cells produce primary cilia longer than those produced elsewhere in the neural tube. Finally, they show that Foxj1 expression in the neural tube attenuates Shh signal transduction by altering cilia structure and modifying the intracellular localisation of the Gli proteins that mediate Shh signalling. Together, these data reveal a novel cilia-dependent mechanism that modulates cellular responses to Shh signalling.
Leaves send mobile signals for size

Organ size in plants and animals is tightly controlled, and partly determined, by cell size and number. Plant leaves, for example, exhibit compensation, in which defective cell proliferation triggers increased postmitotic cell expansion. Now, Hirokazu Tsukaya and colleagues (p. 4221) identify two novel pathways coordinating cell proliferation and expansion in Arabidopsis leaves. Two Arabidopsis mutants, the loss-of-function ANGUSTIFOLIA3 (AN3, a transcriptional co-activator) mutant and the overexpressor KIP-RELATED PROTEIN2 (KRP2, a cyclin-dependent kinase inhibitor) mutant show compensation: in an3 mutant leaves, cell numbers decrease by ~70%, whereas cell size increases by 50%. Using the Cre/lox system, the authors generated leaves chimeric for AN3 and KRP2 expression, and investigated whether compensation occurs in a cell-autonomous or non-cell-autonomous manner. An3-dependent compensation, they report, is indeed non-cell-autonomous and occurs via an intercellular signal restricted to one half of leaves. Conversely, compensation caused by KRP2 overexpression occurs cell-autonomously, possibly via a mitotic cell cycling defect. Future work should shed more light on these events and identify the transmitted signal.
Ongoing Phox2 locks in neuronal differentiation
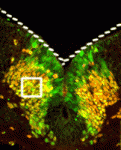
During neuronal differentiation, expression of the transcription factors that determine neuronal identity often continues after their downstream genetic program has been launched. Is this continued expression required for neuronal differentiation? On p. 4211, Jean-François Brunet and colleagues address this question by inactivating the paired-like homeobox genes Phox2a and Phox2b, which specify several classes of visceral neurons, after the developmental timepoint at which they act to initiate visceral neuron differentiation. They report that ongoing Phox2b expression is required in branchiomotor and visceromotor neuronal precursors after their initial specification to maintain their molecular signature, migration pattern and cellular differentiation. Similarly, maintenance of noradrenergic neuron differentiation during embryogenesis requires the ongoing expression of Phox2b in sympathetic ganglia and of Phox2a in the main noradrenergic centre of the developing brain. Thus, neuronal differentiation does not always unfold as a transcriptional ‘cascade’ in which downstream events are irreversibly triggered by an upstream regulator. Instead, as seen here, it sometimes requires continuous input from so-called ‘terminal selector genes’.
Hippo links growth control to tissue homeostasis
Both tissue repair and tissue homeostasis require stem cells that proliferate to replenish lost cells, but the way in which adult stem cells respond to damage and switch between homeostatic and rapid proliferative states is not well understood. In the Drosophila midgut, intestinal stem cells (ISCs) maintain homeostasis, and, in response to damage, can proliferate rapidly following activation of the Jak/Stat pathway. In this issue, two papers demonstrate that Drosophila ISC proliferation, and hence intestinal regeneration, are regulated by the Hippo (Hpo) tumour suppressor pathway, providing an exciting new link between growth control and stem cell proliferation.
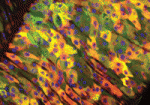
On p. 4147, Nicolas Tapon and colleagues examine the effects of Hpo pathway inactivation in the midgut by overexpressing Yorkie (Yki), a progrowth target that is usually repressed by the Hpo pathway. They report that Yki overexpression in differentiated cells increases ISC proliferation non-cell-autonomously without affecting differentiation, and induces the expression of the Jak/Stat pathway ligand Unpaired. The authors also observe that Yki target genes are induced by bacterial infection, and suggest that the Hpo pathway acts to sense cellular stress within the midgut. Finally, using RNAi, they show that Yki is also required within ISCs to drive proliferation in response to bacterial-induced tissue stress. Based on their findings, they propose that the Hpo pathway is a mediator of the Drosophila midgut regenerative response.
In a second, related paper, Norbert Perrimon and co-workers (p. 4135) demonstrate that Yki overexpression in ISCs induces proliferation cell-autonomously, whereas Yki loss has no effect on ISCs during normal homeostasis. They also show that Yki activity is required in ISCs to mediate the proliferative response to tissue damage, and propose that this effect is elicited by downstream targets that are involved in proliferation and survival. Importantly, they report that, prior to tissue damage, Yki is also repressed by the atypical cadherins Fat and Dachsous, which are upstream components of the Hpo pathway. From their findings, the researchers propose that Yki is inactive under normal homeostasis but becomes activated to induce ISC proliferation when cell-contact cues, and thus Hpo signal transduction, are disrupted by tissue injury.
Also…
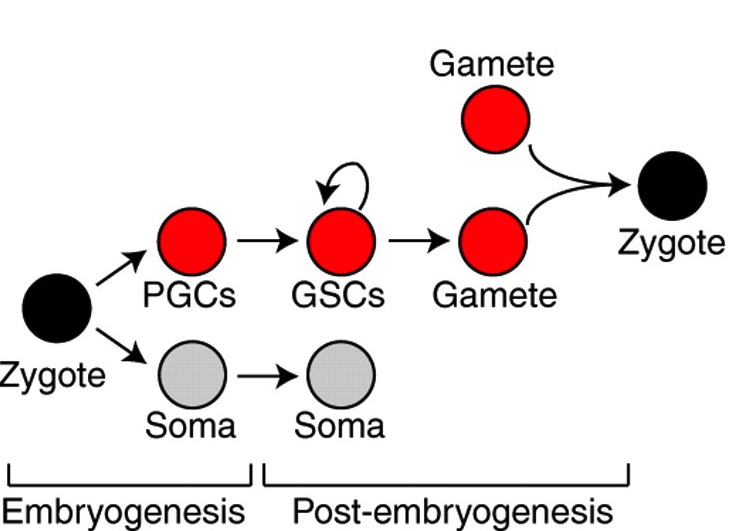
Germline segregation in metazoans can occur during or after embryogenesis and often involves a common set of genes. Juliano, Swartz and Wessel now propose that this gene set represents a conserved germline multipotency programme operating in germ cells and multipotent progenitors.
See the Hypothesis article on p. 4113


 (No Ratings Yet)
(No Ratings Yet)