In Development this week (Vol. 138, Issue 14)
Posted by Seema Grewal, on 21 June 2011
Here are the research highlights from the current issue of Development:
How to make stripes: revising the pair-rules

A key step in Drosophila segmentation is the transition from non-periodic to periodic gene expression patterns, a process that is controlled by transcriptional regulation of the pair-rule genes. Primary pair-rule genes generate their striped expression patterns through stripe-specific cis-regulatory elements that are controlled by the preceding maternal and gap gene expression patterns, whereas secondary pair-rule genes establish their stripe patterns in response to positional cues already provided by the primary pair-rule genes. On p. 3067, Ulrike Gaul and colleagues use computational and experimental approaches to systematically reappraise the complex regulatory architecture that underlies pair-rule stripe formation and, based on their analyses, reclassify fushi tarazu and odd skipped as primary rather than secondary pair-rule genes The researchers also present results that point to a much closer integration of maternal/gap-mediated and pair-rule-mediated regulation than previously recognised and provide new insights into the function of stripe-specific cis-regulatory elements. Together, these results deepen our understanding of periodic pattern generation in the Drosophila embryo.
Sprouty limits cerebellar FGF signals
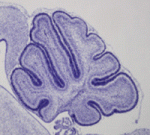
The fibroblast growth factor (FGF) pathway is active in several cell types within the developing cerebellum. During early embryogenesis, FGF signalling helps to establish cerebellar territory but its function during later development is unclear. Now, on p. 2957, M. Albert Basson and colleagues report that the normal development of several cell types in the mouse cerebellum depends on tight regulation of FGF signalling by sprouty genes, which encode feedback antagonists of FGF signalling. Spry1, Spry2 and Spry4 are expressed in the developing cerebellum. The researchers show that simultaneous deletion of multiple sprouty genes results in numerous cerebellar defects, including abnormal folding of cell layers and reduced granule cell proliferation. Reducing the Fgfr1 dosage rescues these abnormalities, confirming that they are due to excess FGF signalling. Moreover, the effects of deregulated signalling on cerebellar morphology depend on the time and cell type in which sprouty genes are deleted. Thus, suggest the researchers, FGF signalling has several distinct functions and must be tightly controlled during cerebellar morphogenesis.
Chemokine evolution and development
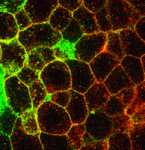
During development, families of ligands and receptors control concurrent processes, but how do cells discriminate between closely related signals? To find out, Erez Raz and co-workers have been studying chemokine signalling during primordial germ cell (PGC) migration in zebrafish embryos (see p. 2909). In vertebrates, the chemokine Cxcl12, which binds the Cxcr4 receptor, guides PGC migration. Zebrafish express two Cxcl12 paralogues and two Cxcr4 receptors. The researchers report that, although PGCs can respond to both Cxcl12 ligands, only Cxcl12a, which exhibits a higher affinity than Cxcl12b for one of the receptors (Cxcr4b), guides the cells. Moreover, a single amino acid exchange switches the relative affinity of the Cxcl12 ligands for the duplicated Cxcr4 receptors, allowing each chemokine to elicit a distinct effect. The researchers suggest that the subfunctionalisation of the cxcl12 genes that followed their duplication occurred through alterations in their expression patterns and in the specificity of receptor binding. Subfunctionalisation of this sort, they suggest, could enable chemokines and other receptor-ligand families to control concurrent developmental processes.
Top-Notch trophoblast vascular invasion
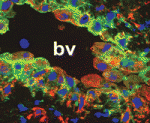
During placental formation, trophoblasts invade and remodel uterine vessels in order to re-route maternal blood to the placenta to nourish the developing embryo. This process fails in pre-eclampsia, a serious but common pregnancy complication. Here (see p. 2987), Susan Fisher and colleagues report that Notch signalling plays a key role in trophoblast endovascular invasion. By immunostaining human placental tissue sections, the researchers show that Notch receptors/ligands are modulated in a stepwise manner during trophoblast invasion. Inhibition of Notch signalling reduces invasion of cultured human trophoblasts and expression of the arterial marker EFNB2. Similarly, in mice, conditional deletion of Notch2 reduces arterial invasion, the size of maternal blood canals and placental perfusion, and leads to litter-wide lethality. Finally, in placental tissue sections obtained from women with pre-eclampsia, expression of the Notch ligand JAG1 is absent in perivascular and endovascular trophoblasts. Together, these results indicate that Notch signalling is crucial for trophoblast vascular invasion and that Notch signalling defects are involved in the pathogenesis of pre-eclampsia.
Getting to the heart of epicardial potential
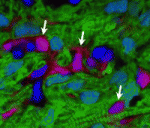
Regenerative medicine could provide treatments for heart disease but a source of cells capable of regenerating cardiac muscle cells remains elusive. One possible source is the epicardium, but lineage-tracing studies have produced conflicting results about the extent to which epicardial cells act as a natural source of cardiac muscle during development. Now, on p. 2895, Kazu Kikuchi and co-workers show that, in zebrafish, epicardial cells adopt only non-myocardial fates during heart development and also during heart regeneration, which is a naturally occurring process in adult zebrafish. The researchers identify the transcription factor gene tcf21 as a specific epicardial marker that is expressed throughout heart development and regeneration. Using tcf21 regulatory sequences and inducible Cre recombinase technology, they show that larval or adult cells labelled by tcf21 expression give rise to adult epicardial and perivascular cells during heart development and regeneration but do not differentiate into cardiomyocytes during either form of cardiogenesis. Thus, in zebrafish, natural epicardial fates are limited to non-myocardial cell types.
How PCP signalling directs neuronal migration
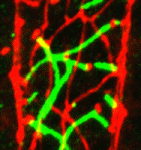
Planar cell polarity (PCP) signalling is implicated in the migration of facial branchiomotor (FBM) neurons during vertebrate brain development but how exactly does it function during this process? Cecilia Moens and colleagues now propose that PCP pathway components and a newly identified protein – Nance-Horan syndrome-like 1b (Nhsl1b) – have essential cell-autonomous functions during neuronal migration in zebrafish (see p. 3033). The researchers identify nhsl1b as a gene required for FBM neuron migration in a forward genetic screen. Nhsl1b localises to FBM neuron membrane protrusions, they report, and interacts with the PCP component Scribble (Scrib) to control FBM neuron migration. In cell transplantation experiments, they show that FBM neuron migration requires the cell-autonomous functions of Nhsl1b, Scrib and the PCP component Vangl2, in addition to the non-cell-autonomous roles of Scrib and Vangl2, which polarise the epithelial cells in the environment of the migrating neurons. The researchers propose, therefore, that Nhsl1b is a neuronal PCP effector that functions in migrating neurons to execute directed cell movements.
Plus…
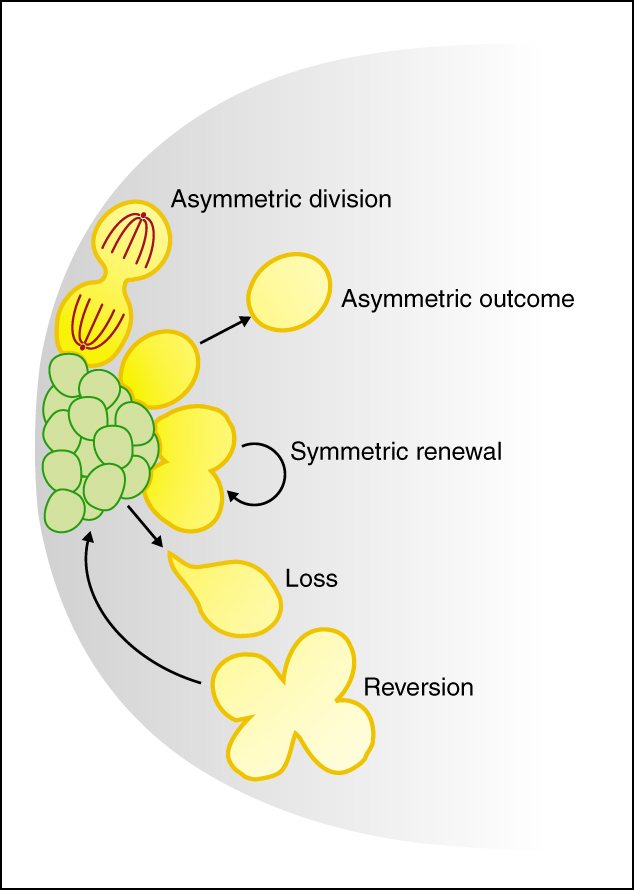 The stem cell niche: lessons from the Drosophila testis
The stem cell niche: lessons from the Drosophila testis
Tissue maintenance depends on stem cells that reside in specialized niches. Here, de Cuevas and Matunis review recent studies of the Drosophila testis and discuss how germline and somatic stem cells within this niche respond to local and systemic changes.
See the Review article on p. 2861
Development of the musculoskeletal system: meeting the neighbors
In March 2011, researchers met for the second Batsheva Seminar on Integrative Perspectives on the Development of the Musculoskeletal System. As reviewed by Gabrielle Kardon, the discussions at this meeting highlighted that interactions between the different tissue components are crucial for musculoskeletal morphogenesis.
See the Meeting Review on p. 2855


 (1 votes)
(1 votes)