In Development this week (Vol. 138, Issue 20)
Posted by Seema Grewal, on 22 September 2011
Here are the highlights from the current issue of Development:
ApoE: a role in neurogenesis
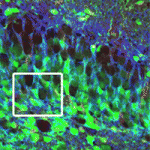
Hippocampal neurodegeneration occurs in most forms of dementia, including Alzheimer’s disease. There is, therefore, intense interest in unravelling the mechanisms that underlie adult neurogenesis in this region of the brain. Now, on p. 4351, Steven Kernie and colleagues report that the cholesterol carrier apolipoprotein E (ApoE) is required for the maintenance of the neural stem/progenitor cell pool in the adult dentate gyrus region of the mouse hippocampus. The researchers show that ApoE is minimally expressed within the neural progenitor pool during early dentate gyrus development, when neural stem/progenitor cells are rapidly proliferating and differentiating into neurons. However, ApoE expression is markedly upregulated in adult dentate gyrus stem/progenitor cells, which proliferate more slowly. Notably, in ApoE-deficient mice, dentate gyrus neural stem/progenitor cells continue to proliferate rapidly, which ultimately depletes the neural stem cell pool. These and other data suggest that ApoE helps to regulate hippocampal progenitor cell fate and provide a mechanism by which human APOE polymorphisms might contribute to late-onset hippocampal neurodegenerative diseases.
A gutsy new model for intestinal development
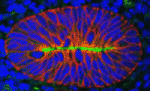
The mechanisms that drive early intestinal development are poorly understood, but it is widely believed that the foetal intestinal epithelium is multilayered (stratified). Here (see p. 4423), Deborah Gumucio and co-workers overturn this dogma. By analysing cell polarity, cell shape and cell dynamics in the foetal mouse intestine, they show that the early intestinal epithelium is single-layered (pseudostratified) and undergoes interkinetic nuclear migration (a process seen in other pseudostratified epithelia, in which nuclei move from the basal to the apical surface of the epithelium during the cell cycle). They report that microtubule- and actinomyosin-dependent apicobasal elongation drives the growth of intestinal epithelium girth that occurs at mid-gestation and that villus formation occurs by expansion of the apical surface. Finally, they show that, as in the pseudostratified neural tube, the actin-binding protein Shroom3 is crucial for the maintenance of the foetal intestinal epithelium. These results suggest a new model for intestinal morphogenesis in which the epithelium remains single-layered and apicobasally polarised throughout early intestinal development.
Excretory systems: regeneration and evolution
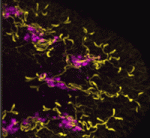
Because planarians can regenerate a complete body from a tissue fragment they present a powerful system in which to study cell, tissue and organ regeneration, and to look for conserved developmental mechanisms. Peter Reddien and colleagues now describe a regulatory programme for the regeneration of the planarian Schmidtea mediterranea excretory system, the protonephridia (see p. 4387). The S. mediterranea protonephridia consists of tubules, which are dispersed throughout the animal’s body, two types of tubule-associated cells, and ciliated terminal cells, which drive filtration from the extracellular space into the tubule lumen. The researchers use RNAi screening assays and microarray analyses to show that Six1/2-2, POU2/3, hunchback, Eya, Sal1 and Osr, which encode transcriptional regulators, are involved in protonephridia regeneration. Notably, apart from hunchback, all these genes are also required for vertebrate kidney development. Moreover, the researchers show that planarian and vertebrate excretory cells express several homologous proteins involved in reabsorption and waste modification. Together, these findings suggest that metazoan excretory systems share a common evolutionary origin.
TPR-GoLoco moves to orientate division
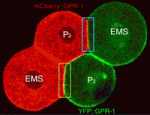
Cell divisions must be correctly oriented, usually through mitotic spindle orientation, to ensure normal development. Extrinsic signals sometimes control division orientation, but how? To address this question, Adam Werts and co-workers have been investigating the localisation of the TPR-GoLoco protein pair GPR-1/2 in C. elegans embryos (see p. 4411). In four-cell stage embryos, GPR-1/2 is enriched at the junction between two cells – the endomesodermal precursor EMS and the germline precursor P2 – and both cells align their division towards this cell-cell contact. The researchers report that, unexpectedly, GPR-1/2 distribution is asymmetric in P2 but not in EMS. Instructive intercellular signalling through MES-1/SRC-1 determines the asymmetric localisation of GPR-1/2 in P2, they report, and this distribution (which is established through GPR-1/2 destabilisation at one cell contact, and its diffusion and stabilisation at another cell contact) is important for normal development. Overall, these results identify the dynamic localisation of GPR-1/2 as a key mediator of cell division orientation in response to external signalling.
Mediator complex sizes up plant development

Final organ size is regulated by coordinated cell proliferation and cell expansion, which control cell number and cell size, respectively. Little is known about how organ size is determined in plants, but now, on p. 4545, Ran Xu and Yunhai Li report that MEDIATOR COMPLEX SUBUNIT 25 (MED25) regulates organ size in Arabidopsis thaliana. The researchers discover this new role for MED25 – a gene that controls shade avoidance and stress responses in Arabidopsis and is involved in transcriptional regulation – through a genetic screen for mutations that enhance the floral size of the da1-1 mutant; DA1 is a negative regulator of seed and organ size that restricts cell proliferation. Loss-of-function mutants in MED25 form large organs, they report, whereas plants overexpressing MED25 have small organs. These alterations in organ size are caused by changes in both cell number and cell size. Thus, the researchers suggest, MED25 acts within the transcriptional machinery to regulate plant organ size by restricting both cell proliferation and cell expansion.
Axons set oligodendrocyte myelinating potential

Myelination facilitates the transmission of electrical signals along axons and ensures their long-term viability, and most axons in the central nervous system are eventually myelinated by oligodendrocytes. But are the timing and extent of myelination regulated by the intrinsic properties of oligodendrocytes or by axons? To address this question, David Lyons and colleagues examine myelination by single oligodendrocytes in vivo in zebrafish (see p. 4443). As in mammals, zebrafish oligodendrocytes myelinate either a few large caliber axons or numerous smaller axons, they report. They further show that the large caliber Mauthner axon is the first axon to be myelinated. Then, using two independent genetic manipulations, the researchers generate zebrafish that have additional Mauthner axons. In these fish, oligodendrocytes that typically myelinate one Mauthner axon in wild-type fish myelinate multiple Mauthner axons, and oligodendrocytes that exclusively myelinate smaller caliber axons in wild-type fish also myelinate the supernumerary Mauthner axons. Thus, the researchers conclude, individual axons regulate the myelinating potential of single oligodendrocytes.
Plus…
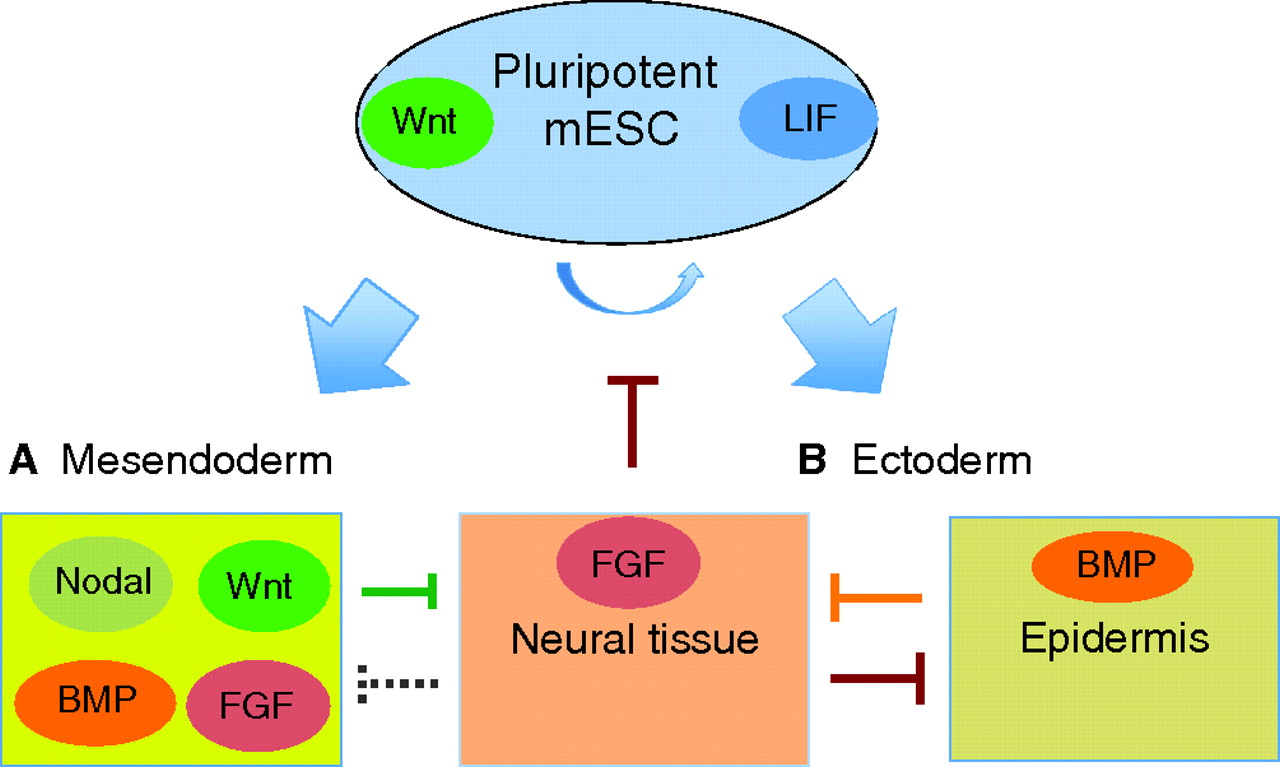
The Wnt signaling pathway controls lineage specification and axial patterning in vertebrate embryos and, as reviewed here by Sergei Sokol, recent studies have uncovered a new mode of Wnt signaling that acts to maintain pluripotency in embryonic stem cells.
See the Review article on p. 4341
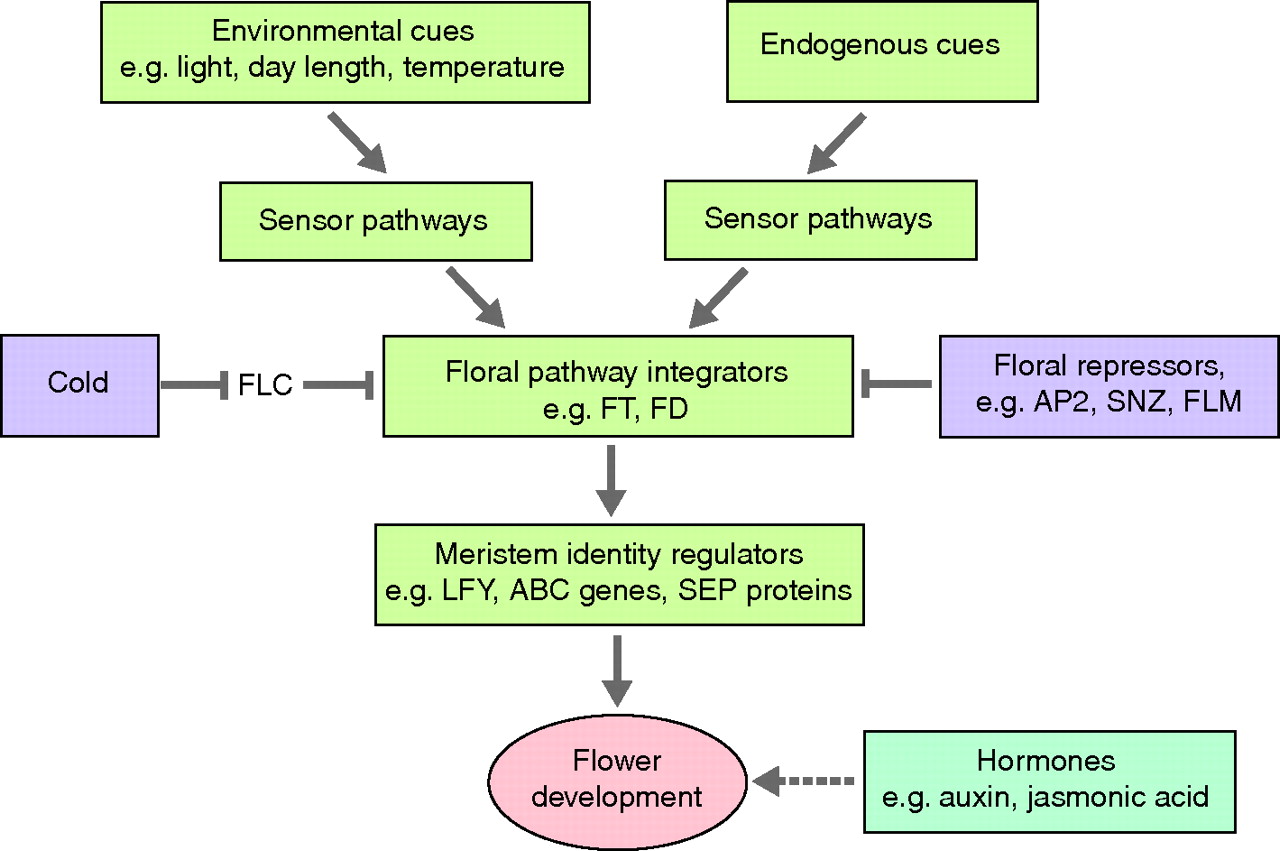
The International Workshop on Molecular Mechanisms Controlling Flower Development took place in Italy in June 2011. As reviewed by Francois Parcy and Jan Lohmann, the results presented at this workshop underlined how mechanistic studies of both model and diverse species are deepening our understanding of the cellular processes involved in flowering.
See the Meeting Review on p. 4335


 (No Ratings Yet)
(No Ratings Yet)