In Development this week (Vol. 139, Issue 11)
Posted by Seema Grewal, on 8 May 2012
Here are the research highlights from the current issue of Development:
Laminin cue for epithelial polarity
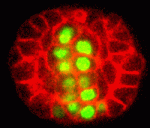
During the development of many animal organs, mesenchymal cells co-ordinately polarize to form epithelial sheets or tubes. In vitro studies have suggested that the extracellular matrix component laminin functions as a polarity cue during this mesenchymal to epithelial transition. Here (p. 2050), Jeffrey Rasmussen and colleagues provide in vivo evidence for laminin’s involvement in polarization by studying the development of the C. elegans pharynx, an epithelial tube that forms from pharyngeal precursor cells (PPCs). The researchers show that cell fate regulators, including the transcription factor PHA-4, cause the PPCs to form a bilaterally symmetric, rectangular array of cells called the ‘double plate’. PPC polarization and apical PAR localisation begin in the double plate cells, which then undergo apical constriction to form a cylindrical cyst. Notably, laminin provides an essential cue that orients the apical localisation of the PAR-3 complex in the double plate but not in the developing C. elegans intestine. Thus, the researchers conclude, laminin is an early polarizing cue for some but not all epithelia.
Why oocytes are predisposed to aneuploidy
During mitosis, the spindle assembly checkpoint (SAC) coordinates proper bipolar chromosome attachment with the anaphase-promoting complex/cyclosome (APC/C)-mediated destruction of cyclin B1 that is required for anaphase onset, thereby avoiding chromosome mis-segregation and aneuploidy. The generation of a Mad2-based signal at kinetochores is central to current models of SAC-based APC/C inhibition: during mitosis, the kinetochores of polar chromosomes (non-aligned bivalents), which are at the greatest risk of mis-segregating, preferentially recruit Mad2, which couples SAC activation to aneuploidy risk. Paradoxically, although an SAC operates in mammalian oocytes, meiosis I is notoriously error prone. Two papers in this issue investigate this long-standing puzzle.
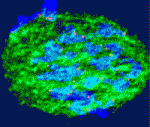
On p. 1947, Keith Jones and colleagues examine the timing of Mad2 loss from mouse oocyte kinetochores. The formation of stable kinetochore-microtubule attachments in mid-prometaphase (3-4 hours before anaphase), they report, coincides with the loss of Mad2 from the kinetochores and the start of APC/C-mediated cyclin B1 destruction. Thus, SAC inhibition of the APC/C ends in mid-prometaphase. However, in a third of oocytes examined, this timing did not coincide with bivalent congression. Notably, the presence of non-aligned bivalents (which were weakly positive for Mad2, under less tension than congressed bivalents, and in the process of establishing correct bi-orientation) did not affect the time between APC/C activation and anaphase onset, and non-aligned bivalents sometimes persisted until anaphase, resulting in homologue non-disjunction. Thus, in oocytes, a few erroneous microtubule-kinetochore attachments may go uncorrected because they do not generate a sufficient SAC ‘wait anaphase’ signal to inhibit the APC/C.
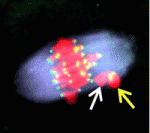
On p. 1941, Liming Gui and Hayden Homer investigate how the SAC responds to polar chromosomes during meiosis I in oocytes. They show that Mad2 is not preferentially recruited to the kinetochores of the rare polar chromosomes that occur in wild-type mouse oocytes or to the kinetochores of the more abundant polar chromosomes that are found in oocytes depleted of the kinesin-7 motor CENP-E. Moreover, in CENP-E-depleted oocytes all the kinetochores eventually become devoid of Mad2, even though the capacity of the chromosomes to form stable attachments to the spindle is severely compromised. These and other findings suggest that SAC signalling is uncoupled from chromosomal position during meiosis I in mouse oocytes, thereby predisposing oocytes to aneuploidy.
Digging out the flowery function of APETALA2
The regulation of gene expression by transcription factors drives cell fate specification during development. In Arabidopsis, transcription factors encoded by four classes of homeotic genes control floral organ identity. The A-class gene APETALA2 (AP2) promotes sepal and petal identity and restricts expression of the C-class gene AGAMOUS (AG), which specifies stamen and carpel identity, but how does AP2 perform these functions? On p. 1978, Xuemei Chen and co-workers report that AP2 recognises and acts through an AT-rich sequence element. The researchers show that AP2R2 (one of two DNA-binding domains in AP2) binds a non-canonical AT-rich target sequence in vitro and that the presence of this sequence in the second intron of AG is important for the restriction of AG expression in vivo. Other experiments indicate that AP2 directly regulates AG expression in young flowers through this sequence element, which is highly conserved in Brassicaceae. Together, these findings shed light on the molecular mechanism underlying AP2 action and provide a missing link in the mechanisms controlling flower development.
Membrane trafficking and epithelial polarity
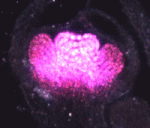
Biological tubes composed of polarized epithelial cells perform many functions in multicellular organisms. The establishment and maintenance of epithelial polarity depend on polarized trafficking of membrane components to the apical or basolateral domains of epithelial cells, but exactly how trafficking regulates epithelial polarity is unclear. In this issue, two papers describe a new and unexpected role for the post-Golgi vesicle coat clathrin and its adaptor AP-1 in apical sorting and lumen formation in the C. elegans intestine.
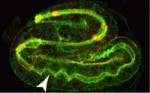
On p. 2061, Grégoire Michaux and colleagues report that the clathrin adaptor AP-1 is required for epithelial polarity in the C. elegans intestine. Depletion of AP-1 subunits does not affect the establishment of epithelial polarity or the formation of the intestinal lumen, the researchers report. However, they show that AP-1 is essential for the apical localisation of the oligopeptide transporter PEPT-1 and the polarity proteins PAR-6 and CDC-42, and for the basolateral distribution of the monocarboxylate transporter SLCF-1. They also show that AP-1 depletion triggers the formation of ectopic apical lumens between intestinal cells along the lateral membranes later during embryogenesis.
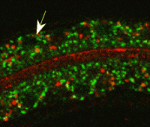
On p. 2071, Verena Gobel and colleagues perform an unbiased RNAi screen for apicobasal polarity and tubulogenesis defects in the C. elegans intestine and identify clathrin and AP-1 as being required for apical polarity and lumen formation. The researchers show that clathrin/AP-1-mediated polarized transport co-operates with a sphingolipid-dependent apical sorting process. Furthermore, they report, the depletion of clathrin, AP-1 or glycosphingolipid biosynthetic enzymes causes a set of apical membrane proteins (including PAR-6) to mislocalise basolaterally and generate ectopic lateral lumens. Finally, they show that clathrin-coated and sphingolipid-rich vesicles assemble at polarized plasma membrane domains in a co-dependent and AP-1-dependent manner.
Together, these findings suggest that clathrin/AP-1 controls both basolateral and apical sorting, an unexpected finding given that, until now, clathrin and its AP-1 adaptor had been thought to regulate only basolateral sorting in mammalian epithelia. Importantly, these findings indicate that this newly discovered clathrin/AP-1 function in apical sorting is required to regulate epithelial polarity in vivo in a tubular epithelium and that the clathrin/AP-1 apical sorting pathway converges with a sphingolipid-dependent apical trafficking path.
Plus…
Tet family proteins and 5-hydroxymethylcytosine in development and disease
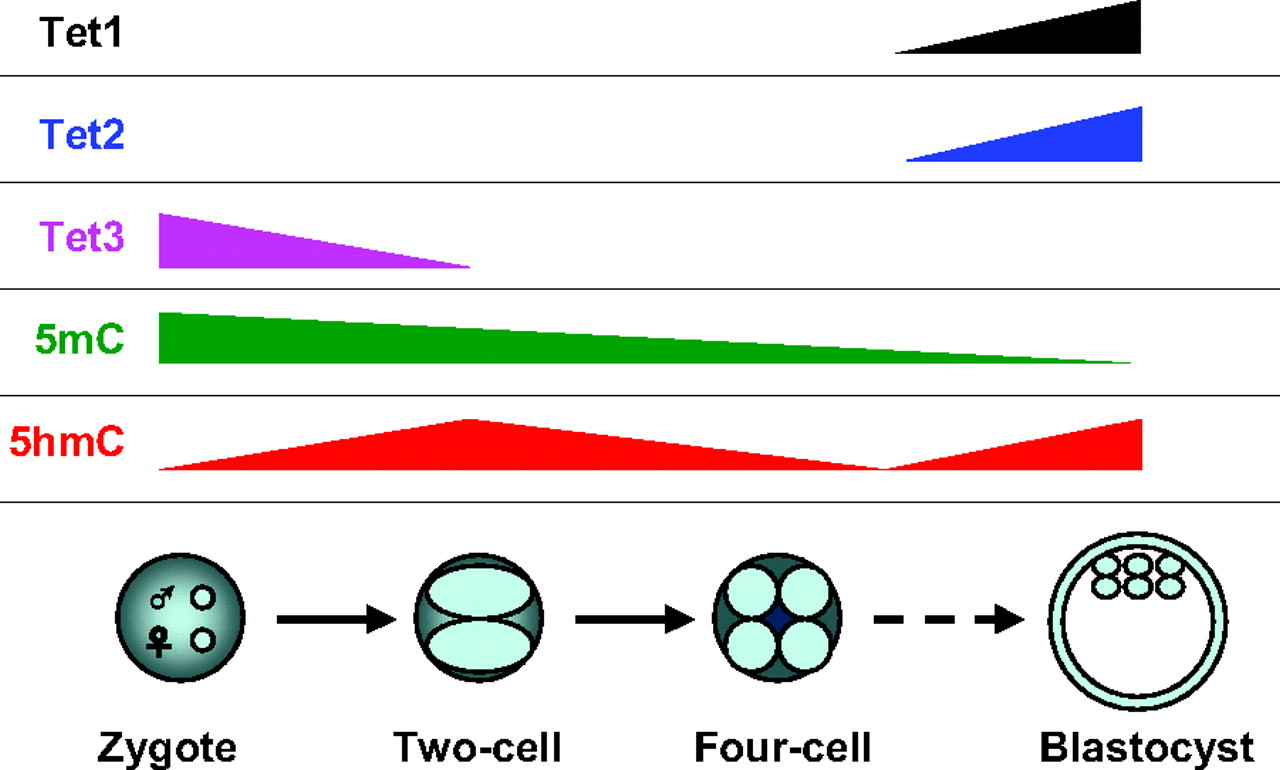
Over the past few decades, DNA methylation at the 5-position of cytosine (5-methylcytosine, 5mC) has emerged as an important epigenetic modification that plays essential roles in development, aging and disease. In this Issue, Tan and Shi provide an overview of the role of Tet family proteins and 5hmC in development and cancer. See the Primer article on p. 1895
25 years of Development!
The Journal of Embryology and Experimental Morphology (JEEM) was founded in 1953, but it wasn’t until 1987, in a bold move by The Company of Biologists and the journal’s editors, that the journal was rebranded, restyled and relaunched as the journal we now know as Development.
We’ll be celebrating celebrating our quarter-century throughout the year, but to kick-start the celebrations we’ve invited past and present Editors in Chief of Development to share their memories and thoughts on their time in charge.
See the Editorials from Chris Wylie, Jim Smith and Olivier Pourquie.


 (No Ratings Yet)
(No Ratings Yet)