In Development this week (Vol. 139, Issue 13)
Posted by Seema Grewal, on 6 June 2012
Here are the highlights from the current issue of Development:
Two-step loss of pluripotency
 During early development, embryonic cells can form derivatives of all three embryonic layers. This pluripotency, which is regulated by a gene regulatory network that includes the transcription factors Oct4 and Nanog, is lost in mouse embryos between about E7.5 and E8.5. Here (p. 2288), Rodrigo Osorno, Anestis Tsakiridis and colleagues investigate the precise timing and mechanism of pluripotency loss in the mouse embryo. Pluripotency, they report, is extinguished at the onset of somitogenesis, and the loss of pluripotency coincides with reduced chromatin accessibility of the regulatory regions of Oct4 and Nanog, and decreased expression of these genes. Notably, pluripotency correlates with threshold levels of Oct4 and, consistent with this observation, the researchers identify a novel non-pluripotent state during which an increase in Oct4 expression can rapidly reverse chromatin closure and restore pluripotency. Finally, the researchers show that this temporary state is followed by permanent methylation-based epigenetic stabilization of the non-pluripotent state. Thus, two mechanistically separate events are responsible for the elimination of pluripotent cells during development.
During early development, embryonic cells can form derivatives of all three embryonic layers. This pluripotency, which is regulated by a gene regulatory network that includes the transcription factors Oct4 and Nanog, is lost in mouse embryos between about E7.5 and E8.5. Here (p. 2288), Rodrigo Osorno, Anestis Tsakiridis and colleagues investigate the precise timing and mechanism of pluripotency loss in the mouse embryo. Pluripotency, they report, is extinguished at the onset of somitogenesis, and the loss of pluripotency coincides with reduced chromatin accessibility of the regulatory regions of Oct4 and Nanog, and decreased expression of these genes. Notably, pluripotency correlates with threshold levels of Oct4 and, consistent with this observation, the researchers identify a novel non-pluripotent state during which an increase in Oct4 expression can rapidly reverse chromatin closure and restore pluripotency. Finally, the researchers show that this temporary state is followed by permanent methylation-based epigenetic stabilization of the non-pluripotent state. Thus, two mechanistically separate events are responsible for the elimination of pluripotent cells during development.
Lateral inhibition at neurogenic wavefronts
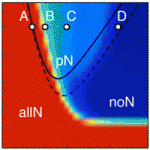 During neurogenesis, lateral inhibition controls the final number of neurons. Neuronal precursors that express high levels of Delta prevent the neuronal differentiation of neighbouring cells by inducing Notch-dependent inhibitory signals in these neighbours. However, neurogenic wavefronts spread through non-neurogenic areas during development, so why isn’t lateral inhibition disrupted where these wavefronts contact non-neurogenic tissue? José María Frade, Saúl Ares and colleagues investigate this puzzle on p. 2321. The researchers show that Delta-like 1 (Dll1) is widely expressed by non-neurogenic precursors at the periphery of the developing chick retina. Using a mathematical model of lateral inhibition, they show that the absence of Dll1 ahead of the neurogenic wavefront reduces the robustness of lateral inhibition, enhances neurogenesis and alters the shape of the neurogenic wavefront, predictions that are consistent with previous observations in the retina of mice in which Dll1 was conditionally mutated. The researchers propose, therefore, that Notch-independent Delta expression ahead of the neurogenic wavefront optimizes neurogenesis by preventing perturbations in lateral inhibition and wavefront progression.
During neurogenesis, lateral inhibition controls the final number of neurons. Neuronal precursors that express high levels of Delta prevent the neuronal differentiation of neighbouring cells by inducing Notch-dependent inhibitory signals in these neighbours. However, neurogenic wavefronts spread through non-neurogenic areas during development, so why isn’t lateral inhibition disrupted where these wavefronts contact non-neurogenic tissue? José María Frade, Saúl Ares and colleagues investigate this puzzle on p. 2321. The researchers show that Delta-like 1 (Dll1) is widely expressed by non-neurogenic precursors at the periphery of the developing chick retina. Using a mathematical model of lateral inhibition, they show that the absence of Dll1 ahead of the neurogenic wavefront reduces the robustness of lateral inhibition, enhances neurogenesis and alters the shape of the neurogenic wavefront, predictions that are consistent with previous observations in the retina of mice in which Dll1 was conditionally mutated. The researchers propose, therefore, that Notch-independent Delta expression ahead of the neurogenic wavefront optimizes neurogenesis by preventing perturbations in lateral inhibition and wavefront progression.
Hedgehog signals modular bone growth
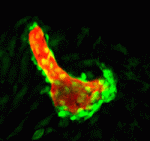 The vertebrate skeleton provides structural support and protection for vital organs but how its component bones acquire their unique shapes is unknown. Here (p. 2371), Charles Kimmel and colleagues investigate the genetic regulation of morphogenesis in dermal bones, which are formed by direct differentiation of mesenchymal cells into osteoblasts, by analyzing the development of the zebrafish opercle. The researchers report that the Hedgehog (Hh) family ligand Indian hedgehog a (Ihha) is specifically expressed in a population of osteoblasts localized along the growing edge of this craniofacial bone. Loss of ihha function reduces pre-osteoblast proliferation and bone growth, whereas hyperactive Hh signalling in mutants for the Hh receptor ptch1 has opposite effects. Time-lapse and live-imaging experiments show that ihha-dependent bone growth is region specific and begins at the start of a second phase of morphogenesis, during which the opercle acquires a more-complex form. These results suggest that dermal bone development is modular, with different genes functioning at specific times and locations to pattern growth.
The vertebrate skeleton provides structural support and protection for vital organs but how its component bones acquire their unique shapes is unknown. Here (p. 2371), Charles Kimmel and colleagues investigate the genetic regulation of morphogenesis in dermal bones, which are formed by direct differentiation of mesenchymal cells into osteoblasts, by analyzing the development of the zebrafish opercle. The researchers report that the Hedgehog (Hh) family ligand Indian hedgehog a (Ihha) is specifically expressed in a population of osteoblasts localized along the growing edge of this craniofacial bone. Loss of ihha function reduces pre-osteoblast proliferation and bone growth, whereas hyperactive Hh signalling in mutants for the Hh receptor ptch1 has opposite effects. Time-lapse and live-imaging experiments show that ihha-dependent bone growth is region specific and begins at the start of a second phase of morphogenesis, during which the opercle acquires a more-complex form. These results suggest that dermal bone development is modular, with different genes functioning at specific times and locations to pattern growth.
Lymphangiogenesis goes lyve1
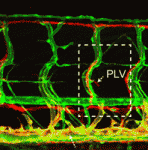 The lymphatic system regulates tissue fluid homeostasis, aids immunity and helps absorb dietary fat. Because aberrant lymphatic growth is associated with cancer metastasis and chronic inflammation, a better understanding of lymphangiogenesis could identify therapeutic targets for these and other lymphatic abnormalities. The major trunk lymphatic vessel in the zebrafish embryo is a well-established model of lymphangiogenesis but the rest of the zebrafish’s lymphatic system is poorly described. On p. 2381, Phil Crosier and colleagues remedy this situation by generating transgenic lines in which the promoter of lyve1 (which encodes lymphatic vessel endothelial hyaluronan receptor 1) drives lymphatic vessel expression of fluorescent reporters. The researchers generate a map of zebrafish lymphatic development and characterize facial, intestinal and lateral lymphatic vessel networks for the first time. They also describe a novel mechanism that underlies the development of the lateral facial lymphatic. These results show that lymphatic vessel formation in zebrafish is more complex than previously thought, thereby increasing the versatility of zebrafish as a model of lymphangiogenesis.
The lymphatic system regulates tissue fluid homeostasis, aids immunity and helps absorb dietary fat. Because aberrant lymphatic growth is associated with cancer metastasis and chronic inflammation, a better understanding of lymphangiogenesis could identify therapeutic targets for these and other lymphatic abnormalities. The major trunk lymphatic vessel in the zebrafish embryo is a well-established model of lymphangiogenesis but the rest of the zebrafish’s lymphatic system is poorly described. On p. 2381, Phil Crosier and colleagues remedy this situation by generating transgenic lines in which the promoter of lyve1 (which encodes lymphatic vessel endothelial hyaluronan receptor 1) drives lymphatic vessel expression of fluorescent reporters. The researchers generate a map of zebrafish lymphatic development and characterize facial, intestinal and lateral lymphatic vessel networks for the first time. They also describe a novel mechanism that underlies the development of the lateral facial lymphatic. These results show that lymphatic vessel formation in zebrafish is more complex than previously thought, thereby increasing the versatility of zebrafish as a model of lymphangiogenesis.
Making dopamine neurons: less Nurr1 later is more
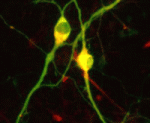 In vitro differentiation of stem cells has the potential to generate specific cell types for clinical use but, to date, this approach has mainly created cells with unsatisfactory phenotypes. Now, Sang-Hun Lee and colleagues generate mature dopamine (DA) neurons from rat neural progenitor cells (NPCs; see p. 2447). Midbrain DA neurons, which are the main source of dopamine in the mammalian nervous system, are lost in Parkinson’s disease. Previous attempts to induce NPC differentiation into DA neurons through the forced expression of Nurr1, a transcription factor that is expressed during midbrain development, induced DA-specific marker expression but failed to generate mature DA neurons. Here, by using an inducible retroviral vector system to express less exogenous Nurr1, and at a later time point than used previously, the researchers generate morphologically and phenotypically mature DA neurons from NPCs. Adjustment of the levels and timings of the expression of cell type-specific transcription factors to match physiological conditions, suggest the researchers, could facilitate the in vitro generation of other useful cell types.
In vitro differentiation of stem cells has the potential to generate specific cell types for clinical use but, to date, this approach has mainly created cells with unsatisfactory phenotypes. Now, Sang-Hun Lee and colleagues generate mature dopamine (DA) neurons from rat neural progenitor cells (NPCs; see p. 2447). Midbrain DA neurons, which are the main source of dopamine in the mammalian nervous system, are lost in Parkinson’s disease. Previous attempts to induce NPC differentiation into DA neurons through the forced expression of Nurr1, a transcription factor that is expressed during midbrain development, induced DA-specific marker expression but failed to generate mature DA neurons. Here, by using an inducible retroviral vector system to express less exogenous Nurr1, and at a later time point than used previously, the researchers generate morphologically and phenotypically mature DA neurons from NPCs. Adjustment of the levels and timings of the expression of cell type-specific transcription factors to match physiological conditions, suggest the researchers, could facilitate the in vitro generation of other useful cell types.
Endoderm conduit for LR signals
 Establishment of the left-right (LR) body axis is a crucial step in embryogenesis. In mouse embryos, a leftward flow of fluid in the node establishes an initial LR signal, which is transferred to the lateral plate mesoderm (LPM) where it triggers the gene expression program responsible for LR asymmetry. But how is the LR signal transferred to the LPM? On p. 2426, Yukio Saijoh and co-workers test the hypothesis that endoderm (which lies next to the node and covers the LPM) is involved in this process. The researchers report that expression of LR asymmetric genes in the left LPM is greatly reduced or absent in most mouse embryos null for the Sox17 transcription factor, which exhibit endoderm-specific defects. Interestingly, membrane localization of gap junction connexin proteins is impaired and intercellular transport between endoderm cells is disrupted in Sox17–/– endoderm. Together, these results suggest that endoderm cells, possibly via gap junction communication, play an essential role in the transfer of LR signals during mouse LR axis establishment.
Establishment of the left-right (LR) body axis is a crucial step in embryogenesis. In mouse embryos, a leftward flow of fluid in the node establishes an initial LR signal, which is transferred to the lateral plate mesoderm (LPM) where it triggers the gene expression program responsible for LR asymmetry. But how is the LR signal transferred to the LPM? On p. 2426, Yukio Saijoh and co-workers test the hypothesis that endoderm (which lies next to the node and covers the LPM) is involved in this process. The researchers report that expression of LR asymmetric genes in the left LPM is greatly reduced or absent in most mouse embryos null for the Sox17 transcription factor, which exhibit endoderm-specific defects. Interestingly, membrane localization of gap junction connexin proteins is impaired and intercellular transport between endoderm cells is disrupted in Sox17–/– endoderm. Together, these results suggest that endoderm cells, possibly via gap junction communication, play an essential role in the transfer of LR signals during mouse LR axis establishment.
Plus…
Tudor domain proteins in development
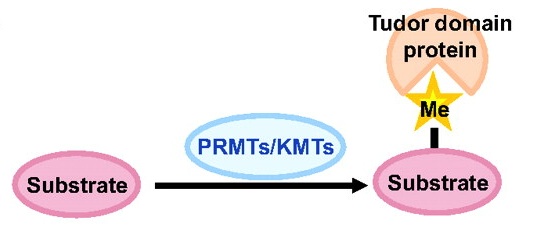 Toshie Kai and colleagues discuss the emerging roles of Tudor domain proteins during development, most notably in the Piwi-interacting RNA pathway, but also in other aspects of RNA metabolism, the DNA damage response and chromatin modification. See the Primer article on p. 2255
Toshie Kai and colleagues discuss the emerging roles of Tudor domain proteins during development, most notably in the Piwi-interacting RNA pathway, but also in other aspects of RNA metabolism, the DNA damage response and chromatin modification. See the Primer article on p. 2255
The Prdm family: expanding roles in stem cells and development
 Prdm factors either act as direct histone methyltransferases or recruit a suite of histone-modifying enzymes to target promoters. In their review, Hohenauer and Moore discuss the roles played by these proteins in stem cells and throughout development. See the Review article on p. 2267
Prdm factors either act as direct histone methyltransferases or recruit a suite of histone-modifying enzymes to target promoters. In their review, Hohenauer and Moore discuss the roles played by these proteins in stem cells and throughout development. See the Review article on p. 2267


 (No Ratings Yet)
(No Ratings Yet)