In Development this week (Vol. 140, Issue 16)
Posted by Seema Grewal, on 30 July 2013
Here are the highlights form the current issue of Development:
Nf2 regulates neural progenitor proliferation
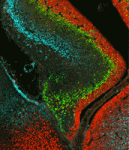 Mutation of neurofibromatosis 2 (NF2) results in nervous system tumours. Molecularly, Nf2 has diverse functions, regulating cell-cell junction formation and various signalling pathways, including the Hippo-Yap pathway. However, the roles of Nf2 in the nervous system, and how its loss promotes tumorigenesis, are poorly understood. Here (p. 3323), Xinwei Cao and co-workers analyse the consequences of Nf2 deletion in the dorsal telencephalon. Although the mutant mice are viable, they display significant brain malformations associated with neural progenitor cell (NPC) hyperproliferation. To determine how Nf2 limits NPC expansion, the authors performed a microarray analysis and found many known targets of the transcriptional coactivator Yap upregulated upon Nf2 deletion, suggesting that Nf2 may inhibit Yap activity. Consistent with this, protein levels and nuclear localization of Yap and its paralog Taz are increased in Nf2 mutants. Moreover, Yap deletion rescues the Nf2 mutant phenotype – demonstrating the functional importance of this regulation. These data uncover a key role for Nf2 and Yap/Taz in regulating NPC proliferation in the developing brain.
Mutation of neurofibromatosis 2 (NF2) results in nervous system tumours. Molecularly, Nf2 has diverse functions, regulating cell-cell junction formation and various signalling pathways, including the Hippo-Yap pathway. However, the roles of Nf2 in the nervous system, and how its loss promotes tumorigenesis, are poorly understood. Here (p. 3323), Xinwei Cao and co-workers analyse the consequences of Nf2 deletion in the dorsal telencephalon. Although the mutant mice are viable, they display significant brain malformations associated with neural progenitor cell (NPC) hyperproliferation. To determine how Nf2 limits NPC expansion, the authors performed a microarray analysis and found many known targets of the transcriptional coactivator Yap upregulated upon Nf2 deletion, suggesting that Nf2 may inhibit Yap activity. Consistent with this, protein levels and nuclear localization of Yap and its paralog Taz are increased in Nf2 mutants. Moreover, Yap deletion rescues the Nf2 mutant phenotype – demonstrating the functional importance of this regulation. These data uncover a key role for Nf2 and Yap/Taz in regulating NPC proliferation in the developing brain.
Staying in sync through development
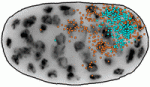 During embryogenesis, transcriptional regulation must be coordinated with growth and cell division, so that genes are turned on or off in the right cells at the right time. Arjun Raj and colleagues now investigate the coupling of gene expression and cell division in C. elegans (p. 3385). They find that global retardation of development by temperature change or gene mutation slows down the cell cycle, and this is accompanied by a similar delay in expression of particular developmental genes – so the synchrony between cell cycle and gene expression is retained. These findings suggest that transcription might be directly cell cycle dependent. However, mutations that cause cell cycle delays in specific lineages uncouple cell division and transcription, arguing against the onset of transcription being tied to a particular division cycle. Conversely, it is known that cell division in C. elegans embryos proceeds independently of zygotic transcription. Together, these data demonstrate that cell proliferation and gene expression are well synchronised, but raise the key question of how this synchrony is achieved.
During embryogenesis, transcriptional regulation must be coordinated with growth and cell division, so that genes are turned on or off in the right cells at the right time. Arjun Raj and colleagues now investigate the coupling of gene expression and cell division in C. elegans (p. 3385). They find that global retardation of development by temperature change or gene mutation slows down the cell cycle, and this is accompanied by a similar delay in expression of particular developmental genes – so the synchrony between cell cycle and gene expression is retained. These findings suggest that transcription might be directly cell cycle dependent. However, mutations that cause cell cycle delays in specific lineages uncouple cell division and transcription, arguing against the onset of transcription being tied to a particular division cycle. Conversely, it is known that cell division in C. elegans embryos proceeds independently of zygotic transcription. Together, these data demonstrate that cell proliferation and gene expression are well synchronised, but raise the key question of how this synchrony is achieved.
How cilia know which way to point
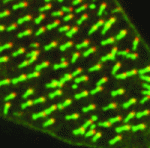 Cells lining the lumen of various organs, such as the lung airway and the female reproductive tract, are multiciliated, and all the cilia are oriented in the same direction to generate flow. But how is cilia orientation coordinated within cells and across tissues? Chris Kintner and colleagues use the epithelial cells of Xenopus embryos as a model to study multicilate cell differentiation. On p. 3468, they identify a new regulator of cilia polarisation, the coiled-coil protein bbof1. Bbof1 is expressed in multicilate cells and localises to the axoneme and the basal body – the structure that determines cilia orientation. Upon bbof1 depletion, motile cilia still form, but are unable to generate significant flow because their orientation is disturbed. Notably, bbof1 is not required for the initial phase of cilia polarisation, but rather for the later refinement step, and for stabilising the alignment. Although the mechanism by which bbof1 acts remains unclear, this work identifies a key factor regulating cilia orientation and function.
Cells lining the lumen of various organs, such as the lung airway and the female reproductive tract, are multiciliated, and all the cilia are oriented in the same direction to generate flow. But how is cilia orientation coordinated within cells and across tissues? Chris Kintner and colleagues use the epithelial cells of Xenopus embryos as a model to study multicilate cell differentiation. On p. 3468, they identify a new regulator of cilia polarisation, the coiled-coil protein bbof1. Bbof1 is expressed in multicilate cells and localises to the axoneme and the basal body – the structure that determines cilia orientation. Upon bbof1 depletion, motile cilia still form, but are unable to generate significant flow because their orientation is disturbed. Notably, bbof1 is not required for the initial phase of cilia polarisation, but rather for the later refinement step, and for stabilising the alignment. Although the mechanism by which bbof1 acts remains unclear, this work identifies a key factor regulating cilia orientation and function.
Go with the flow: circulating BMP promotes endothelial quiescence
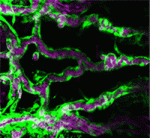 Blood flow through the developing vasculature regulates vessel formation – both via the distribution of endocrine factors, and via mechanical force-induced responses. Several signalling pathways are known to be involved in this process, including signalling via the TGFb receptor Alk1, whose activity promotes quiescence in newly formed arteries and whose expression is itself dependent upon blood flow. On p. 3403, Beth Roman and colleagues demonstrate that not only Alk1 expression but also its activity are dependent upon blood flow in developing zebrafish. They identify Bmp10 as the endogenous ligand for Alk1 in this context, and find that Bmp10 is exclusively expressed in the heart, and not in the vascular tissue. Through elegant experiments using embryos in which the heart has been stopped but alk1 expression restored, they show that Bmp10 injection can locally rescue Alk1 pathway activity and downstream transcriptional responses. Thus, their data suggest that blood flow is required to distribute cardiac-derived Bmp10 into the vasculature, where it activates Alk1 to promote quiescence in endothelial cells.
Blood flow through the developing vasculature regulates vessel formation – both via the distribution of endocrine factors, and via mechanical force-induced responses. Several signalling pathways are known to be involved in this process, including signalling via the TGFb receptor Alk1, whose activity promotes quiescence in newly formed arteries and whose expression is itself dependent upon blood flow. On p. 3403, Beth Roman and colleagues demonstrate that not only Alk1 expression but also its activity are dependent upon blood flow in developing zebrafish. They identify Bmp10 as the endogenous ligand for Alk1 in this context, and find that Bmp10 is exclusively expressed in the heart, and not in the vascular tissue. Through elegant experiments using embryos in which the heart has been stopped but alk1 expression restored, they show that Bmp10 injection can locally rescue Alk1 pathway activity and downstream transcriptional responses. Thus, their data suggest that blood flow is required to distribute cardiac-derived Bmp10 into the vasculature, where it activates Alk1 to promote quiescence in endothelial cells.
Histone methylation: not so dynamic after all
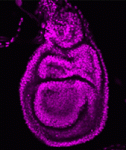 Polycomb group proteins are chromatin regulators with highly conserved functions. The Polycomb repressive complex 2 (PRC2) methylates H3K27 to stably silence target genes, including the HOX genes in Drosophila. More recently, Utx and Jmjd3 demethylases were found to reverse PRC2-mediated H3K27 methylation, and it has been suggested that a dynamic cycle of methylation and demethylation is required for appropriate regulation of gene expression. Now, Ömer Copur and Jürg Müller challenge this view (p. 3478), via the analysis of Drosophila Utx mutants. Lack of zygotic Utx function has no effect on Drosophila development, although mutant adults die shortly after hatching. Loss of both maternal and zygotic Utx, however, leads to larval death and to defects in HOX gene expression – in both the embryo and larval imaginal discs. Thus, it appears that Utx in Drosophila – and, by inference, H3K27 demethylation – is required only at early stages to set up the patterns of HOX expression; it is largely dispensable later in development, suggesting that H3K27 methylation may in fact be very stable.
Polycomb group proteins are chromatin regulators with highly conserved functions. The Polycomb repressive complex 2 (PRC2) methylates H3K27 to stably silence target genes, including the HOX genes in Drosophila. More recently, Utx and Jmjd3 demethylases were found to reverse PRC2-mediated H3K27 methylation, and it has been suggested that a dynamic cycle of methylation and demethylation is required for appropriate regulation of gene expression. Now, Ömer Copur and Jürg Müller challenge this view (p. 3478), via the analysis of Drosophila Utx mutants. Lack of zygotic Utx function has no effect on Drosophila development, although mutant adults die shortly after hatching. Loss of both maternal and zygotic Utx, however, leads to larval death and to defects in HOX gene expression – in both the embryo and larval imaginal discs. Thus, it appears that Utx in Drosophila – and, by inference, H3K27 demethylation – is required only at early stages to set up the patterns of HOX expression; it is largely dispensable later in development, suggesting that H3K27 methylation may in fact be very stable.
An integral role for integrin β1 in the pancreas
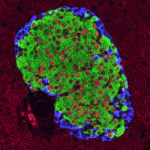 Integrins mediate cell-matrix adhesion and are also capable of inducing intracellular signalling cascades to regulate cell proliferation, differentiation and other cell behaviours. In vitro, disruption of β1 integrin function has been shown to affect various aspects of pancreatic β-cell activity. On p. 3360, Vincenzo Cirulli and co-workers analyse the consequences of deleting β1 integrin in β-cells in vivo in mice. The mutant mice have smaller pancreatic islets that exhibit matrix adhesion defects when cultured in vitro. Notably, cell proliferation is severely impaired in the mutant β-cells, and the expression of cell cycle regulators is highly abnormal. However, these cells are able to differentiate properly and to express insulin, and are glucose responsive; in fact, they show increased levels of insulin and the mutant mice show no signs of diabetes. These results highlight differences between the ascribed functions of β1-integrin in vitro versus in vivo and define its key role in promoting proliferation during pancreatic islet development.
Integrins mediate cell-matrix adhesion and are also capable of inducing intracellular signalling cascades to regulate cell proliferation, differentiation and other cell behaviours. In vitro, disruption of β1 integrin function has been shown to affect various aspects of pancreatic β-cell activity. On p. 3360, Vincenzo Cirulli and co-workers analyse the consequences of deleting β1 integrin in β-cells in vivo in mice. The mutant mice have smaller pancreatic islets that exhibit matrix adhesion defects when cultured in vitro. Notably, cell proliferation is severely impaired in the mutant β-cells, and the expression of cell cycle regulators is highly abnormal. However, these cells are able to differentiate properly and to express insulin, and are glucose responsive; in fact, they show increased levels of insulin and the mutant mice show no signs of diabetes. These results highlight differences between the ascribed functions of β1-integrin in vitro versus in vivo and define its key role in promoting proliferation during pancreatic islet development.
PLUS…
Adult neural stem cells: plastic or restricted neuronal fates?
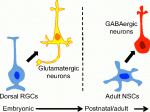 Eduardo Sequerro and colleagues review studies of postnatal and adult neurogenesis, challenging the notion that fixed genetic programs restrict neuronal fate. They hypothesize that the adult brain maintains plastic neural stem cells that are capable of responding to changes in environmental cues and generating diverse neuronal types. See the Hypothesis article on p. 3303
Eduardo Sequerro and colleagues review studies of postnatal and adult neurogenesis, challenging the notion that fixed genetic programs restrict neuronal fate. They hypothesize that the adult brain maintains plastic neural stem cells that are capable of responding to changes in environmental cues and generating diverse neuronal types. See the Hypothesis article on p. 3303
Clustered protocadherins
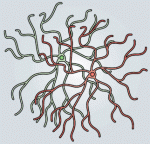 Weisheng Chen and Tom Maniatis provide a concise overview of the molecular and cellular biology of clustered Pcdhs, highlighting how they generate single cell diversity in the vertebrate nervous system and how such diversity may be used in neural circuit assembly. See the Development at a Glance poster article on p. 3297
Weisheng Chen and Tom Maniatis provide a concise overview of the molecular and cellular biology of clustered Pcdhs, highlighting how they generate single cell diversity in the vertebrate nervous system and how such diversity may be used in neural circuit assembly. See the Development at a Glance poster article on p. 3297


 (No Ratings Yet)
(No Ratings Yet)